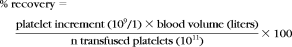Visual scoring versus histogram subtraction of in vivo binding of immunoglobulins against platelets after transfusion
Abstract
BACKGROUND
We developed a technique, in vivo binding of immunoglobulins in the platelet immunofluorescence test (IVBI-PIFT), that detects immunoglobulins bound in vivo to transfused platelets. The visually scored results of this technique, however, are susceptible to interobserver variation. We describe a more objective method to generate results in IVBI-PIFT.
METHODS
We studied 201 samples in 120 patients with hematologic malignancies in the IVBI-PIFT. Histogram subtraction, i.e., fluorescence (anti–immunoglobulin G and fluorescein isothiocyanate) histogram before platelet transfusion subtracted from the histogram after platelet transfusion, was compared with visual scoring (pattern 1: no enhanced fluorescence before and after transfusion; pattern 2: enhanced fluorescence before and after platelet transfusion; pattern 3: enhanced fluorescence before transfusion; pattern 4: enhanced fluorescence after transfusion, interpreted as alloimmunization). After histogram subtraction, the number of remaining events (events post substraction, EPS) and the mean amount of fluorescence of these remaining events (mean channel post substraction, MCPS) were used and compared with the visual scoring and with platelet survival after transfusion.
RESULTS
In 26 (13%) of the 201 samples studied in the IVBI-PIFT, fewer than three of five observers agreed on the visually scored pattern. In the 175 (87%) remaining samples, histogram subtraction showed a significant differentiation between pattern 4 and patterns 1 and 2 by using EPS, whereas patterns 4 and 3 were distinguished by using MCPS. The combination of EPS and MCPS differentiated best between pattern 4 and patterns 1, 2, and 3 (73% sensitivity, 96% specificity, 79% positive predictive value, and 95% negative predictive value). In contrast, the predictive value for platelet recovery after 1 and 16 h of pattern 4 from the visual scoring method and the results of histogram subtraction were poor.
CONCLUSION
This objective method of histogram subtraction correlated well with the visual scoring method of IVBI-PIFT. © 2003 Wiley-Liss, Inc.
Patients who receive platelet transfusions are at risk of alloimmunization against HLA antigens or platelet-specific antigens (1, 2). These antibodies may interfere with the survival of the transfused platelets (3, 4) and may cause febrile transfusion reactions. The presence of alloantibodies against platelet and leucocyte antigens are routinely measured by incubation of sera of patients with a panel of donor cells (platelets, mononuclear cells, and granulocytes). Several methods have been described to detect the presence of these alloantibodies: the lymphocytotoxic test (5-7), the microscopic platelet suspension immunofluorescence test (8), the enzyme-linked immunosorbent assay (9-12), the monoclonal antibody-specific immobilization of platelet antigens assay (13-15), the monoclonal 125I-labeled anti-immunoglobulin (Ig) G assay (16), flow cytometric platelet and lymphocyte immunofluorescence tests (17-21), 51Cr platelet lysis assay (22), and platelet radioactive antiglobulin test (23). Although the sensitivity of these methods may differ, all are based on the binding of antibodies under in vitro circumstances and to panel cells. In vivo circumstances, e.g., platelet-bound immune complexes or drug-dependent antibodies that also may adversely affect platelet survival, are not evaluated by these tests. Testing against panel cells rather than against platelets of the transfused donor(s), incubation under in vitro circumstances, and the inability to detect platelet-bound immunoglobulins not related to alloantibody binding may be technical causes for the discrepancies that are found between the laboratory results and platelet recovery after transfusion in the clinic. We previously described a test that detects IgG bound to (transfused) platelets in vivo, the in vivo binding of immunoglobulins in the platelet immunofluorescence test (IVBI-PIFT) (24). In this work, the results of the IVBI-PIFT were visually scored by a single blinded observer. We demonstrated that preferential binding of immunoglobulins after platelet transfusion (pattern 4) is associated with significantly lower platelet recoveries than when no binding of immunoglobulins after platelet transfusion occurs (pattern 1). The introduction of the IVBI-PIFT in our clinic showed a considerable interobserver variability for the visual scoring of the four different patterns. We evaluate the use of histogram subtraction as an objective alternative for the subjective visual comparison of paired histograms and correlate the results of both methods of data interpretation with the corresponding platelet recoveries at 1 and 16 h after transfusion.
MATERIALS AND METHODS
Samples
From October 1994 through August 2000, 201 paired serum samples (before and after transfusion) were studied in 120 random patients (mean, 1.7 transfusions/patient; range, 1–7) who were treated for a malignant lymphoma or leukemia in our hospital. Patients received a platelet transfusion of random donors prophylactically (a) when platelet count was less than 10 × 109/l, (b) before an intervention, or (c) because of clinically manifest bleeding at higher platelet counts. The serum samples were collected before and 1 h after platelet transfusion.
IVBI-PIFT
The IVBI-PIFT method was described elsewhere (24). In short, after collection of whole blood, before and after platelet transfusion, the results of three platelet suspensions after incubation with a mixture of fluorescein-conjugated goat anti-IgG were used (a) before transfusion, (b) the transfusion bag, and (c) 1 h after transfusion. For every suspension, a histogram showing the distribution of IgG fluorescence on a logarithmic scale was obtained by collecting four-parameter flow cytometric data from 8,000 platelets. For each transfusion, an overlay histogram was composed from the fluorescence of platelet suspensions I, II, and III. All tests were performed in duplicate.
Histogram Subtraction
The histograms of the IgG fluorescence of suspension I (before transfusion) was subtracted from the histogram of suspension III (after transfusion). This was done by subtracting the number of platelets for each fluorescence channel separately (linear scale of 256 histogram channels). If differences between the corresponding channels of histograms I and III resulted in negative values, these were replaced by zero events in the subtraction histogram (Fig. 1). Histogram II was used as a negative control (see below). This left a subtraction histogram that could be characterized by the number of remaining events (= total number of platelets of all fluorescence channels after this subtraction) and the mean fluorescence of the remaining events. First, events post subtraction (EPS) was defined as the average of all remaining events of both (duplicate) subtraction histograms. Second, mean channel post subtraction (MCPS) was defined as the average of the mean channel values of both (duplicate) subtraction histograms.
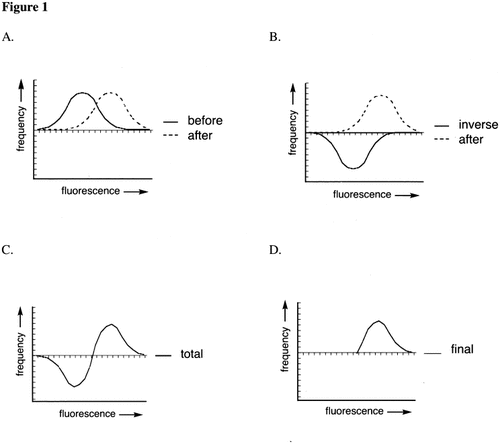
Stepwise deduction of the histogram before transfusion from the histogram after transfusion. A: Both curves in one figure. B: Curve before transfusion is inverted with respect to the x axis. C: Addition of the number of events per fluorescence range. D: The negative part of the curve is removed.
Visual Scoring (Fig. 2)
Patterns were scored independently and blindly by five observers according to the following definitions:
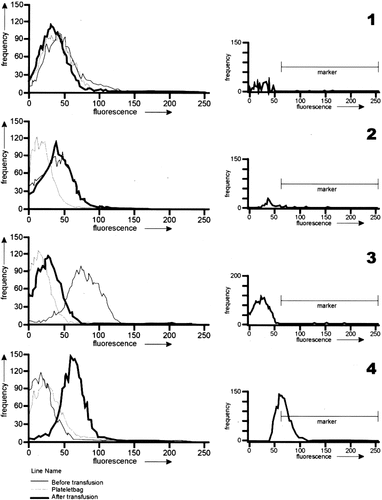
Visual scoring and histogram subtraction of IVBI-PIFT. 1–4: The four patterns observed in IVBI-PIFT by visual scoring (left) and the results of the histogram subtraction, after platelet transfusion minus before platelet transfusion (right).
Pattern 1: similar shapes of histograms of suspensions I, II, and III (interpretation: no alloimmunization)
Pattern 2: strongly resembling shifts to the right of both histograms of suspensions I and III relative to suspension II (interpretation: binding of IgG not related to alloimmunization)
Pattern 3: a shift to the right of the histogram of suspension I relative to suspension III (interpretation: preferential binding of IgG to own or previously transfused platelets)
Pattern 4: a shift to the right of the histogram of suspension III relative to suspension I (interpretation: alloimmunization; Fig. 2) (24).
When three or more of all five observers indicated the same pattern for both (duplicate) overlay histograms per platelet transfusion, this was considered the consensus pattern and was used for further analysis.
Platelet Transfusion Results
Statistical Methods
EPS and MCPS as continuous variables were compared with the consensus patterns 1, 2, 3, and 4 by using the Kruskal-Wallis test. Pearson's chi-square test or Fisher's exact test, whichever was appropriate, was used to compare EPS with MCPS, after dichotomization into the lower two-thirds and the higher one-third, with the four consensus patterns. Univariate logistic regression was used to calculate P values for differences in recovery for the visual and histogram subtraction methods, with adjustment for multiple transfusions in single patients. All P values were two sided, and P ≤ 0.05 was considered statistically significant.
RESULTS
Visual Scoring Versus EPS and MCPS Results
In 201 transfusions, the results of IVBI-PIFT were visually scored and generated by histogram subtraction. In 26 (13%) of the transfusions studied in the IVBI-PIFT, fewer than three of five observers agreed on the pattern, so no consensus was reached. The comparison of the histogram subtraction results with the visually scored patterns of IVBI-PIFT therefore was performed in 175 transfusions administered to 111 patients.
As expected, the median number of EPS was significantly higher for transfusions showing pattern 4 (alloimmunization) than for those showing patterns 1 and 2 (no alloimmunization; 1,480, 615, and 566, respectively; Fig. 3A), but no difference was found with transfusions showing pattern 3, for which the median EPS was 1,222. The median value of MCPS was significantly higher in pattern 4 than in pattern 3, with values of 49 and 7, respectively (Fig. 3B), but no difference was found with transfusions showing patterns 1 and 2, with MCPS values of 23 and 46, respectively. Dichotomization of the transfusions into a upper one-third (n = 58) with an EPS of at least 1,040 and the lower two-thirds (n = 117) with an EPS lower than 1,040 showed the following correlations (Table 1). The proportion of transfusions with an EPS of at least 1,040 was significantly higher for patterns 3 and 4 than for patterns 1 and 2 (i.e., 52 of 72, or 72%, vs. 6 of 103, or 6%, transfusions, respectively; P < 0.0001). The difference between patterns 3 and 4, on the one hand, and patterns 1 and 2, on the other, is caused by the fact that the positions of the data in histograms I and III are divergent for patterns 3 and 4 and, to a large extent, overlapping for patterns 1 and 2 (Fig. 2). In contrast, the proportion of transfusions with an MCPS of at least 37 was clearly higher for pattern 4 than for pattern 3 (i.e., 22 of 30, 73%, vs. 3 of 42, 7%, transfusions, respectively; P < 0.0001). This difference is caused by the fact that the post-transfusional histogram (III) is situated far more to the right than the pre-transfusional histogram (I) in pattern 4, whereas the reverse is true for pattern 3 (Fig. 2).
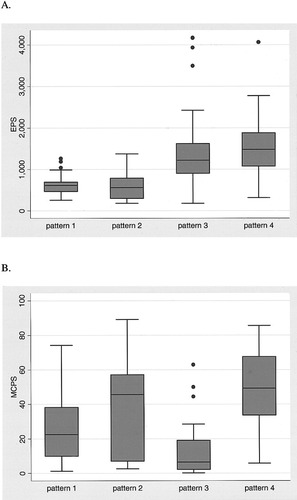
Box-and-whisker plot of histogram subtraction versus the visual scoring method. The line in the middle of the box represents the median of the data. The box extends from the 25th percentile (x25) to the 75th percentile(x75), the so-called interquartile range (IQR). The lines emerging from the box are called whiskers, and they extend to the upper and lower adjacent values. The upper adjacent value is defined as the largest data point ≤ x75 + 1.5 × IQR. The lower adjacent value is defined as the smallest data point ≥ x25 − 1.5 × IQR. Observed points more extreme than the adjacent values, if any, are individually plotted. A: EPS versus patterns 1–4. B: MCPS versus patterns 1–4.
| Pattern 1 (n = 82) | Pattern 2 (n = 21) | Pattern 3 (n = 42) | Pattern 4 (n = 30) | Total (N = 175) | |
|---|---|---|---|---|---|
| EPS* | |||||
| <1,040 | 79 | 18 | 14 | 6 | 117 |
| ≥1,040 | 3 | 3 | 28 | 24 | 58 |
| MCPS† | |||||
| <37 | 60 | 10 | 39 | 8 | 117 |
| ≥37 | 22 | 11 | 3 | 22 | 58 |
- * P < 0.0001 for pattern 3 and 4 versus patterns 1 and 2.EPS, number of remaining events.
- † P < 0.0001 for pattern 3 versus pattern 4. MCPS, mean amount of fluorescence of the remaining events.
Visual Scoring Versus EPS Combined With MCPS
A combination of EPS and MCPS was used to generate greater distinction between a positive or a negative test result. The proportion of transfusions that demonstrated an EPS of at least 1,040 and an MCPS of at least 37 (these values were described above after dichotomization) was significantly higher for pattern 4 (63%) than for pattern 1 (2%), 2 (14%), and 3 (5%; P < 0.001; Table 2). This resulted in a sensitivity of 63%, a specificity of 95%, a positive predictive value of 73%, and a negative predictive value of 93% in differentiating pattern 4 from patterns 1, 2, and 3. The combination of EPS and MCPS was investigated further to find a cutoff point that showed the best combination of sensitivity, specificity, positive predictive value, and negative predictive value. The best cutoff point was found in the combination of an EPS of at least 1,100 and an MCPS of at least 30, resulting in a sensitivity of 73%, a specificity of 96%, a positive predictive value of 79%, and a negative predictive value of 95% for differentiating pattern 4 from patterns 1, 2, and 3 (Table 2).
| Pattern 1 (n = 82) | Pattern 2 (n = 21) | Pattern 3 (n = 42) | Pattern 4 (n = 30) | Total (N = 175) | |
|---|---|---|---|---|---|
| EPS ≥ 1,040 and MCPS ≥ 37; P < 0.001 | |||||
| No | 80 | 18 | 40 | 11 | 149 |
| Yes | 2 | 3 | 2 | 19 | 26 |
| EPS ≥ 1,100 and MCPS ≥ 30; P < 0.001 | |||||
| No | 80 | 19 | 40 | 8 | 147 |
| Yes | 2 | 2 | 2 | 22 | 28 |
- * Two combinations of cutoff points for EPS and MCPS versus patterns 1, 2, 3 and 4. EPS, number of remaining events; MCPS, mean amount of fluorescence of the remaining events; no, all other transfusions; yes, number of transfusions showing EPS ≥ 1,040 (1,100) and MCPS ≥ 37 (30).
Visual Scoring and Histogram Subtraction Versus Platelet Recovery (Table 3)
The visual scoring method demonstrated no larger proportion of poor platelet recoveries after 1 and 16 h for pattern 4 compared with the recoveries of patterns 1, 2, and 3 (P = 0.89 and P = 0.95, respectively). The proportion of poor platelet recoveries after 1 and 16 h of all patterns differed significantly (P = 0.02) and with borderline significance (P = 0.06), respectively. This was caused mainly by the correlation of pattern 2 with poor platelet recovery after 1 and 16 h.
| Recovery after 1 ha | Recovery after 16 hb | |||
|---|---|---|---|---|
| ≥20%, n (%) | <20%, n (%) | ≥10%, n (%) | <10%, n (%) | |
| Visual scoring | ||||
| Pattern 1 | 42 (51) | 40 (49) | 50 (64) | 28 (36) |
| Pattern 2 | 2 (11) | 19 (89) | 7 (35) | 13 (65) |
| Pattern 3 | 21 (50) | 21 (50) | 27 (75) | 9 (25) |
| Pattern 4 | 13 (43) | 17 (57) | 18 (62) | 11 (38) |
| Histogram subtraction results (EPS ≥ 1,100 and MCPS ≥ 30)c | ||||
| No | 66 (45) | 81 (55) | 84 (61) | 53 (39) |
| Yes | 12 (43) | 16 (57) | 18 (69) | 8 (31) |
- a Recovery after 1 h: P = 0.02 for all patterns, P = 0.89 for pattern 4 versus other patterns, P = 0.85 for histogram subtraction.
- b Recovery after 16 h: P = 0.06 for all patterns, P = 0.95 for pattern 4 versus other patterns, P = 0.42 for histogram subtraction. After 16 h, 12 recovery results were missing: four for pattern 1, one for pattern 2, six for pattern 3, and one for pattern 4; 10 for negative histogram subtraction and two for positive histogram subtraction.
- c EPS, number of remaining events; MCPS, mean amount of fluorescence of the remaining events; no, all other transfusions; yes, number of transfusions showing EPS ≥ 1,040 (1,100) and MCPS ≥ 37 (30).
Subsequently, a positive result of the histogram subtraction (EPS ≥ 1,100 and MCPS ≥ 30) did not demonstrate an association with poor platelet recovery after 1 and 16 h (P = 0.85 and P = 0.42, respectively).
DISCUSSION
Of all platelet transfusions, 24% to 44% do not result in the expected platelet increment (3, 27). Because platelet transfusion failure is often multi-causal in patients with hematologic diseases, a test that reliably detects alloimmunization can be a step forward in selecting patients in need of HLA-matched or cross-matched platelet transfusions. We recently reported that pattern 4 of the IVBI-PIFT significantly correlates with a low recovery of a subsequent platelet transfusion in patients at risk for alloimmunization (24). The results of IVBI-PIFT, however, were visually scored, a method subject to interobserver variation.
In this study, the interobserver variation of the visually scoring of the patterns, defined as fewer than three of five observers reporting the same pattern, was 13% (26 serum samples), which was in accordance with our experience in clinical practice. To study the association of the histogram subtraction with the visual scoring method, we compared the results of histogram subtraction with the “gold standard” visual scoring method, in which three or more of the five observers agreed on the visually scored pattern, and excluded these 26 transfusions from further study. We can not exclude the possibility that this exclusion was selective.
To objectify the results of IVBI-PIFT, histogram subtraction was introduced by subtracting the fluorescence histogram of a serum sample before transfusion from the fluorescence histogram after platelet transfusion. We previously studied the effect of subtracting the mean or median of the histograms. This did not lead to a reliable substitution of the visual scoring method (data not published) because of biphasic curves before or after transfusion or because of a substantial difference in the range of the channel numbers with events before or after transfusion. Because of these difficulties, we studied the subtraction of the number of events per channel number in 175 samples in which consensus could be reached by the visual scoring method. This histogram subtraction resulted in the variables EPS and MCPS. The EPS of at least 1,040 clearly demonstrated a significant differentiation between patterns 3 and 4, on the one hand, and between patterns 1 and 2, on the other. Subsequently, patterns 3 and 4 could be differentiated by an MCPS of at least 37.
We set out to generate a positive or negative test result of IVBI-PIFT by combining the EPS and MCPS. When the combination of the dichotomization cutoff for EPS and MCPS was used (EPS ≥ 1,040 and MCPS ≥ 37), a good differentiation between pattern 4 and patterns 1, 2, and 3 could be achieved. Other cutoff points were tested, which resulted in a best cutoff point characterized by an EPS of at least 1,100 and an MCPS of at least 30. This cutoff point showed a better sensitivity, positive predictive value, and negative predictive value than did the original cutoff point while maintaining the same specificity. Nevertheless, eight (27%) transfusions demonstrating pattern 4 were negative in the histogram subtraction method, and six (4%) transfusions demonstrating pattern 1, 2, or 3 were positive in the histogram subtraction method. This cutoff point has no universal meaning because it is sensitive to laboratory-dependent factors such as temperature, incubation time, laser emitting wavelength, and fluorescence antibody concentration, so it should be validated for each laboratory.
To validate the technique, the results of the visual scoring method and the histogram subtraction method were compared with platelet recovery after 1 and 16 h of the studied platelet transfusion. No significant correlation could be demonstrated between pattern 4 of the visual scoring method and recovery after 1 and 16 h. In contrast, pattern 2 demonstrated a significant negative correlation with platelet recovery after 1 h and after 16 h, but the numbers were small. This may indicate the importance of immune complexes as a causative agent in enhanced destruction of transfused platelets. Pattern 4, defined as increased IgG present on transfused platelets, was interpreted as compatible with alloimmunization and may be used to select patients for HLA-matched platelet transfusions. However, in the present study, we demonstrated that binding of IgG to transfused platelets does not necessarily lead to enhanced donor platelet destruction. The majority of discrepancies between test results and platelet recovery likely were caused by nonimmunologic factors causing enhanced platelet destruction present at the time of transfusion (28).
In conclusion, we demonstrated that histogram subtraction of the IVBI-PIFT by combining EPS and MCPS is a reliable and objective substitution for visually scored consensus patterns. In this study, however, no correlation was demonstrated between pattern 4 of the visual scoring method or a positive histogram subtraction result and platelet recovery after 1 and 16 h.
Acknowledgements
We thank Karola van Rooyen for her help with the layout of the figures.