Sodium pump subunit NKAα1 protects against diabetic endothelial dysfunction by inhibiting ferroptosis through the autophagy-lysosome degradation of ACSL4
Xue-Xue Zhu, Jia-Bao Su, Fang-Ming Wang, and Xiao-Ying Chai contributed equally.
Abstract
The sodium pump Na+/K+-ATPase (NKA), an enzyme ubiquitously expressed in various tissues and cells, is a critical player in maintaining cellular ion homeostasis. Dysregulation of α1 subunit of NKA (NKAα1) has been associated with cardiovascular and metabolic disorders, yet the exact role of NKAα1 in diabetes-induced endothelial malfunction remains incompletely understood. The NKAα1 expression and NKA activity were examined in high-glucose (HG)-exposed endothelial cells (ECs) and mouse aortae, as well as in high-fat-diet (HFD)-fed mice. Acetylcholine (Ach) was utilised to assess endothelium-dependent relaxation (EDR) in isolated mouse aortae. We found that both NKAα1 protein and mRNA levels were significantly downregulated in the aortae of HFD-fed mice, and HG-incubated mouse aortae and ECs. Gain- and loss-of-function experiments revealed that NKAα1 preserves EDR by mitigating oxidative/nitrative stresses in ECs. Overexpression of NKAα1 facilitated EC viability, migration, and angiogenesis by inhibiting the overproduction of superoxide and peroxynitrite. Mechanistically, dysfunctional NKAα1 impaired autophagy process, and prevented the transfer of acyl-CoA synthetase long-chain family member 4 (ACSL4) to the lysosome for degradation, thereby resulting in lipid peroxidation and ferroptosis in ECs. Induction of ferroptosis and inhibition of the autophagy-lysosome pathway blocked the protective effects of NKAα1 on EDR. Eventually, we identified Hamaudol as a potent activator of NKAα1 by restraining the phosphorylation and endocytosis of NKAα1, restoring EDR in obese diabetic mice. Overall, NKAα1 facilitates the autophagic degradation of ACSL4 via the lysosomal pathway, preventing ferroptosis and oxidative/nitrative stress in ECs. NKAα1 may serve as an attractive candidate for the management of vascular disorders associated with diabetes.
Key points
- NKAα1 downregulation impairs endothelial function in diabetes by promoting oxidative/nitrative stress and ferroptosis.
- NKAα1 supports lysosomal degradation of ACSL4 via autophagy, preventing lipid peroxidation and ferroptosis.
- Hamaudol, an activator of NKAα1, restores endothelial relaxation in diabetic mice by inhibiting NKAα1 phosphorylation and endocytosis.
1 INTRODUCTION
Endothelial cells (ECs), located in the endothelium of blood vessels, govern vascular tone by secreting nitric oxide (NO), and prostacyclin (PGI2), with NO being the primary vasodilator.1, 2 In contrast, ECs also release superoxide anion, endothelin 1, and constrictive prostaglandins that lead to vascular contraction.3 Disruption of the balance between vasodilatory and vasoconstrictive factors leads to increased oxidative stress, inflammatory response, and reduced NO bioavailability in ECs, ultimately causing endothelial dysfunction.4 Endothelial dysfunction is a pathological characteristic in a host of human diseases, resulting in functional disruption of multiple organs.4 Restoring EC function has thus been proposed as a promising strategy for treating obesity, diabetes, hypertension, and atherosclerosis.5 Diabetes is becoming a serious global public issue with a rising prevalence globally.6 Patients with diabetes are at a higher risk of cardiovascular accidents.7, 8 Although the mechanisms behind this increased risk are unclear, endothelial dysfunction is a central player in diabetic complications.9 As such, improvement of endothelial dysfunction is of clinical relevance in diabetes.
Na+/K+-ATPase (NKA), a multifunctional ion transporter, maintains sodium and potassium ion balance at the cellular membrane.10 As a widely expressed protein, NKA plays essential roles in sodium/potassium homeostasis and signal transduction in the cardiac, neurological, hepatic, and renal systems.11 This enzyme comprises α, β, and γ subunits.11 To date, four isoforms of NKA subunits have been identified. The NKAα1 isoform, the most prevalent, is found in nearly all cell types.12 The NKAα2 isoform is mainly expressed in skeletal muscle and adipose tissue.12 The NKAα3 isoform is present mainly in neurons and cardiac cells, while the NKAα4 isoform is exclusively expressed in testes.12 Studies have shown that genetic inhibition of NKAα1 leads to metabolic disorders in the liver,13 doxorubicin-induced cardiotoxicity,14 stress-induced anxiety,15 Parkinson disease,16 and brain ischemic injury.17 In the vascular system, inhibition of NKA with ouabain contributes to the increased vascular tone and hypertension in rats.18, 19 Activation of NKA is required for NO-dependent acetylcholine relaxation in porcine internal mammary artery20 and rat aorta.21 Activation of NKA is documented to prevent aortic endothelial dysfunction in lead-treated rats.22 Collectively, it is likely that NKA is critically involved in regulating vascular tension. Recently, it has been established that targeting NKAα1 prevents doxorubicin-induced cardiotoxicity or Parkinson's disease by suppressing the process of ferroptosis.14, 16 We hypothesise that NKAα1 may maintain endothelial function and prevents ferroptosis in ECs since ferroptosis is a crucial contributor to endothelial dysfunction in diabetes.23, 24 Also, we hypothesised that activating NKAα1 may restore endothelial function, reduce oxidative/nitrative stress, and serve as a therapeutic approach for diabetes-related vascular complications. Thereafter, the current study aims to explore whether NKAα isoforms were changed in diabetic endothelial dysfunction, and to investigate whether restored impaired NKAα isoforms protected against diabetes-induced endothelial dysfunction, and to shed light on the underlying mechanisms involved, especially ferroptosis.
2 MATERIALS AND METHODS
2.1 Reagents
NKAα1 antibody (sc-514614) and NKAα3 antibody (sc-376967), NKAα1 siRNA (sc-36010) and control siRNA (sc-36869) were obtained from Santa Cruz. Primary antibodies against β-actin (81115-1-RR), NKAα2 (16836-1-AP), gp91phox (82872-1-RR), ACSL4 (22401-1-AP), Cathepsin B (12216-1-AP), and LAMP1 (67300-1-Ig) were sourced from Proteintech Group. Primary antibodies against GPX4 (ab125066), p62 (ab109012), pan-cadherin (ab51034), LGALS3 (ab2785), LC3B (ab192890), and P-NKAα1 (ab194532) were procured from Abcam. EdU cell proliferation imaging kit containing Alexa Fluor ™ 488 dye (C10337), and TUNEL detection kit, Alexa Fluor ™ 594 dye (C10618) were obtained from Thermo Fisher Scientific. NKAα1 human clone plasmid (RC201009) and scrambled plasmid (GE100003) were bought from ORIGENE. 4′,6-Diamidine-2′-phenylindole dihydrochloride (DAPI) (C1002) and dihydroethidium (DHE) (S0063) were gained from Beyotime Biotechnology. 3-MA (5142-23-4), FerroOrange (SCT210), sodium nitroprusside (BP453), acetylcholine (PHR1546), chloroquine (C6628), phenylephrine (Phe, P1240000), D-glucose (PHR1000), and cycloheximide (CHX, 66-81-9) were purchased from Sigma. Ferrostatin-1 (Fer-1, HY-100579), Z-VAD-fmk (HY-16658B), Z-YVAD-fmk (HY-P1009), necrostatin-1 (Nec-1, HY-15760), and Tetrathiomolybdate (HY-128530) were acquired from MedChemExpress. The kits for triglyceride (TG, A110-1-1), total cholesterol (TC, A111-1-1), and insulin (H203-1-2) were purchased from Jiancheng Institute (Nanjing, China). The selected concentrations of chloroquine (100 µM), Nec-1 (10 µM), Z-VAD-fmk (25 µM), MG-132 (10 µM), Fer-1 (5 µM), Z-YVAD-fmk (10 µM), or Tetrathiomolybdate (1 µM) were based on the previous reports.13, 25
2.2 Animals
All experiments in mice were reviewed and approved by Animal Care and Use Committee of the China Pharmaceutical University (202101017). The animals were kept in an environment with a stable temperature. NKAα1 heterozygous (KAα1+/−) mice were provided by University of Cincinnati.26 Wile-type (WT) C57BL/6J mice and KAα1± mice, aged of 8 weeks, were subjected to a high-fat diet (HFD, 60% kcal as fat, Research Diets) for continuous 12 weeks as described previously.13, 27, 28 Age-matched control mice were fed a normal chow diet for the same duration. Mice received intravenous injections of AAV5-TIE1-NKAα1 overexpression plasmid (1011 viral genome particles for each mouse) or negative control AAV5 vectors after 6 weeks of HFD feeding. After six weeks, the aortae were harvested for functional analyses. Euthanasia was performed by anaesthetising the animals with 5% isoflurane. For ex vivo experiments, the aortic rings (2 mm) were isolated from 8-week-old WT and NKAα1+/− mice and cultured in Dulbecco's modified Eagle medium (DMEM, Gibco, 11960044) coupled with 10% fetal bovine serum (Gibco, 10099–141C), penicillin (100 IU/mL) and streptomycin (100 µg/mL). High-glucose (HG) conditions were constructed by the presence of glucose (25 mM), whereas mannitol (25 mM) was applied as the normal glucose (NG) osmotic control. After 48 h, the aortic rings were mounted onto a myograph for the assessment of vascular tone. Six weeks after tail vein injection of AAV5-TIE1-NKAα1 overexpression plasmids (1011 viral genome particles per mouse) in normal mice, the mouse aortae were then incubated with NG or HG for additional 48 h to evaluate the effects of endothelial cell-specific overexpression of NKAα1 on vascular functions in diabetes.
2.3 Vascular function measurement
After sacrifice, the isolated mouse thoracic aortae were placed in an oxygenated, ice-cold physiological saline solution (PSS). The mouse aortae were then cut into 2-mm rings and mounted on stainless steel wires (Model 620 M, Aarhus, Denmark) containing 5 mL of aerated PSS at 37°C. Arterial segments were stretched to a tension of 3 mN and equilibrated for 60 min before the experiments. Following equilibration, the mouse vascular rings were precontracted with 1 µM phenylephrine (Phe) and subsequently rinsed. Endothelium-dependent relaxation (EDR) was measured by adding various concentrations of acetylcholine (Ach, 10−9 to 10−5 M) to Phe-precontracted rings. Endothelium-independent relaxation was evaluated by applying different doses of sodium nitroprusside (SNP, 10−9 to 10−5 M) to rings where the endothelium was removed by gentle rubbing.
2.4 Western blotting
Proteins from cells and mouse aortae were isolated using prechilled lysis buffer (P0013, Beyotime Biotechnology, Shanghai, China). The protein concentrations in each sample were measured with a BCA kit (P0012S, Beyotime Biotechnology, Shanghai, China) after centrifugation at 12 000 rpm for 15 min at 4°C. Equal protein amounts were separated by SDS-PAGE, and transferred to PVDF membranes (03010040001, Millipore), and blocked with 5% skim milk for 1 h. Primary antibodies were added into the PVDF membranes overnight at 4°C. After incubation of HRP-conjugated secondary antibodies for 1 h at room temperature, the blots were obtained using enhanced chemiluminescence (BL523B, Biosharp).
2.5 Quantitative real-time (RT) PCR
Total RNA in each sample was isolated with Trizol reagents (Vazyme, R711-01) following the manufacturer's protocols. Subsequently, 1 µg of RNA per sample was reversely transcribed using Hifair® III first Strand cDNA Synthesis SuperMix (Yeasen, 11120ES60). Real-time PCR was carried out on an Applied Biosystems QuantStudio 5 PCR system (ThermoFisher, USA), and mRNA levels were normalised to the housekeeping genes with the aid of the 2−∆∆Ct method. Detailed information for the used primer sequences was provided in Tables S1 and S2.
2.6 Measurement of NKA activity
The activity of NKA was measured as we previously depicted.29 Samples were randomly divided into two 50 µL homogenate aliquots. One sample was incubated with the reaction buffer without ouabain, and the other sample with buffer containing 2 mM ouabain. The reactions were initiated with 1 mM ATP, stopped with 10 µL of 100% trichloroacetic acid for 60 min. Free phosphates were measured at 650 nm using a spectrophotometric assay kit.
2.7 Cell culture
Primary endothelial cells (ECs) from mouse aortae were conducted as previously documented.2, 28, 30, 31 Briefly, mice were anaesthetised with 5% isoflurane. Heparin (100 U/mL) was injected into the left ventricle. Mouse aortae were incubated in DMEM with .8 mg/mL collagenase type II (Sigma) for 8 min at 37°C. Detached ECs were collected by centrifugation (1000 rpm, 5 min), re-suspended in DMEM with 20% fetal bovine serum, and cultured in endothelial growth medium (Pricella, CM-M075) until confluency. Primary vascular smooth muscle cells and vascular fibroblasts were cultured as previously described.32-34 Human umbilical vein endothelial cells (HUVECs, CL-0675, Pricella, Wuhan, China) were cultured in RPMI 1640 medium (Gibco, 11875093) supplemented with fetal bovine serum (10%), 100 U/mL penicillin, and 100 µg/mL streptomycin at 37°C and 5% CO2. To simulate diabetic conditions, HUVECs were treated for 24 h with high-glucose/high-fat (HG/HF) medium containing 25 mM glucose (Sigma) and 500 µM palmitate (Sigma) following the established protocols.35, 36
2.8 Cell counting kit-8 (CCK-8) assay
Cell viability was determined using the commercial CCK-8 kits (Beyotime, C0038) following the manufacturer's protocols. Treated cells were seeded in 96-well plates, incubated with the stock CCK-8 solution (10 µL) for 2 h at 37°C, and the absorbance was assessed at 450 nm using a microplate reader.
2.9 DHE staining
The intracellular ROS levels were quantified using the DHE probe. In brief, treated cells were challenged by DHE probe (10 µM) at 37°C for 30 min under the dark environment. After that, the images were captured by a fluorescence microscope (Zeiss, Germany).
2.10 Determination of glutathione (GSH) contents
Intracellular GSH levels were assessed with the aid of a commercial kit (Oxis International Inc., BIOXYTECH GSH-400) In short, samples were lysed in 5% metaphosphoric acid, centrifuged at 12 000 × g for 15 min, and the samples were then collected. GSH levels were measured at 400 nm using a microplate reader.
2.11 Determination of malondialdehyde (MDA)
Lipid peroxidation was quantified by using a commercial MDA assay kit (A03-1-2, Nanjing Jiancheng Bioengineering Institute), where MDA reacts with TBA at 95°C under acidic conditions to form a pink adduct. Absorbance was measured at 450 nm, and MDA concentration calculated per the manufacturer's instructions.
2.12 Determination of Fe2+ levels
Intracellular Fe2+ levels were measured using the FerroOrange probe (DOJINDO, F374). Briefly, treated cells were rinsed twice with sterilised PBS before incubation with the Hoechst (H342, DOJINDO) for 10 min. After that, cells were stained with FerroOrange solution (1:1000 dilution) in the dark at 37°C for 30 min with 5% CO2. The cells without washing were analysed using a fluorescence microscope (Zeiss, Germany). In addition, Fe2+ levels were also measured using commercial kits from Abcam (ab83366).37, 38 A total of 100 µL of iron assay buffer was added and homogenised cells or tissues on ice. The supernatant in the mixture was obtained after centrifugation at 16 000 × g for 10 min. A total of 50 µL of each sample were added to 96-well plates and adjusted to 100 µL using iron assay buffer. For divalent iron assays, 5 µL of buffer was then added, and for total iron assays, 5 µL of iron reducer. After a 30-min incubation at 37°C, 100 µL of Iron Probe was added, followed by another 60-min incubation. The absorbance was measured at 593 nm using a microplate reader, and iron concentration was calculated from a standard curve.
2.13 Mass spectrometry (MS) analysis of lipidomics
Lipids from approximately 5 × 10⁶ cells were extracted following the Bligh and Dyer method.39 After drying, the organic phase was re-dissolved in 50 µL of mobile phase A [trichloromethane/isopropanol 2:1 (v/v)] and vortexed. The phospholipids were then combined with mobile phase B (acetonitrile/isopropanol/water 7:9:4, v/v/v) containing 10 mM ammonium acetate. MS and MS/MS analyses were conducted using a Q-Exactive quadrupole-orbitrap mass spectrometer (Thermo Fisher, Waltham, MA, USA).
2.14 Detection of superoxide anions
The production of superoxide anions, indicative of oxidative stress, was quantified in aortic segments or ECs using lucigenin-enhanced chemiluminescence assay as we previously outlined.40 The contents of superoxide anions were expressed in relative light units (RLU) per milligram of protein per second (RLU/s/mg).
2.15 Measurement of total NOx and nitrotyrosine
Basal NOx (NO, NO2⁻, and NO3⁻) production in cells or mouse aortae was measured by collecting supernatants and using a NO assay kit (Beyotime Biotechnology, S0023) following the manufacturer's protocols. Protein concentration was calculated with a BCA protein assay kit. Nitrotyrosine levels, indicative of protein nitration, were quantified using a Nitrotyrosine Assay Kit (Millipore, 17–376RF) and the results were expressed as pM per mg protein (pM/mg protein).
2.16 Measurement of caspase 3 activity
The caspase-3 activity assay kit (#5723, Cell Signaling Technology) was utilised to measure the activity of caspase-3 in compliance with the manufacturer's indications.41 After washing, fluorescence was detected using an ELISA plate reader with excitation at 380 nm and emission at 450 nm. The activity of caspase-3 was calculated based on the fluorometric signal (excitation/emission = 380/450 nm) and expressed as fold-change compared to the normal group.
2.17 5-Ethynyl-2′-deoxyuridine (EdU) incorporation analysis
HUVEC proliferation was also examined using the EdU cell proliferation kit containing Alexa Fluor ™ 488 dye following the manufacturer's protocols. After treatment, HUVECs were incubated with 50 mM EdU for 2 h, then fixed, permeabilised, and stained with Apollo for 30 min. Nuclear DNA was counterstained with Hoechst 33342, and fluorescent images were captured using a microscope.
2.18 Terminal deoxynucleotidyl transferase-mediated dUTP nick end-labelling (TUNEL) analysis
The apoptosis in HUVECs was assessed by using the TUNEL detection kit in conjunction with Alexa Fluor ™ 594 dye. The treated cells were fixed with 4% paraformaldehyde, permeabilised using .1% Triton X-100, and washed with PBS. After a 1-h incubation at 37°C with TF3-dUTP, nuclei were stained with Hoechst, and TUNEL-positive cells were captured via a fluorescence microscopy.
2.19 Transwell migration assay
HUVEC migration was assessed using a transwell chamber (Corning, CLS3422). Cells (3 × 104/well) were seeded in the upper chamber with serum-free DMEM, while the lower chamber containing DMEM with 10% fetal bovine serum. After 24 h, nonmigrated cells were removed with cotton swabs, and migrated cells were stained with crystal violet (.1%). The number of migrated cells was captured and counted under a phase-contrast microscope (80i, Nikon, Japan).
2.20 Tube formation assay
Ice-cold Matrigel (BD Biocoat, 356234) was added to the required wells of a 96-well plate at 37°C for 30 min. HUVECs (1 × 104 cells/well) were then seeded. The tube formation was captured in each well using a microscope (Olympus, Japan). ImageJ software was utilised for experimental analysis of tube formation, such as the branching points formed by endothelial cells, average tube length, total tube length, and average area of the annular structure.
2.21 Immunofluorescent staining in HUVECs
Immunofluorescent staining was used to localise nitrotyrosine in HUVECs. The fixed cells were incubated with an anti-3-nitrotyrosine antibody (Abcam, ab314438) overnight. After washing, cells were probed with Alexa Fluor™ 594-conjugated Goat anti-Rabbit IgG for 1 h. For double staining, primary antibodies against ACSL4, LGALS3, or LAMP1 were applied overnight, followed by the indicated secondary antibodies. Nuclei were re-stained with DAPI (5 µM) for 5 min, and images were captured with an Olympus microscope (Japan).
2.22 Measurement of eNOS activity
The eNOS activity in ECs and mouse aortae was measured by calculating the transformation of L-arginine to NO using a commercial kit (S0025, Beyotime Biotechnology, Shanghai, China), as previously described.42, 43
2.23 Enzyme-linked immunosorbent assay (ELISA)
The levels of TNF-α (EK0525, EK0527), IL-1β (EK0392, EK0394), VCAM-1 (EK0537, EK0538), and MCP-1 (EK0441, EK0568) in cells and mouse serum were measured using commercial ELISA kits (BOSTER, Wuhan, China).44, 45 Samples, diluted standards, or blanks were added to 96-well plates coated with specific antibodies, and HRP-conjugated secondary antibodies were incubated at 37°C for 30 min. The reactions were stopped with a stop solution, and the absorbance was assessed at 450 nm via a microplate reader (SYNERGY/H4, BioTek, Vermont, USA).
2.24 Cell transfection
Cells were seeded to achieve an optimal density of 80–90% confluence. Cell transfection was performed using Lipofectamine 3000 reagent (Thermo Fisher Scientific, L3000075) with control siRNA, NKAα1 siRNA, empty vectors, or NKAα1 overexpression plasmid. After transfection, the cells were incubated for 6 h before replacing the medium with complete medium. After transfection for 24 h, the cells were then subjected to the indicated treatments. ECs with NKAα1 overexpression and suppression were confirmed by Western blotting.
2.25 Detection of autophagy
Autophagy monitoring was performed as we previously described.13 In short, HUVECs were transfected with a plasmid expressing EGFP-LC3 using Lipofectamine 3000 to monitor autophagy flux. The LC3 dots were photographed using a laser confocal microscope (Fluoview FV1000, Olympus).
2.26 Molecular docking
The protein structure of NKAα1 (PDB: 7E1Z) was optimised using MOE 2019 software. The molecular structure of Hamaudol was retrieved from the PubChem database and imported into Chem3D. For binding affinity assessment, the Hamaudol ligands and protein files were uploaded separately to SwissDock for molecular docking. After optimising the configuration using MM2 force field, semi flexible docking was performed using MOE 2019 software, and the docking results were evaluated using the London dG scoring function. Two-dimensional (2D) interaction predictions between NKAα1 and Hamaudol were performed using MOE 2019 software.
2.27 Extraction of cell membrane and cytoplasmic proteins
Membrane and cytoplasmic proteins were extracted using a commercial protein extraction kit (P0033, Beyotime Biotechnology). Samples were incubated with 1 mL of membrane protein extraction reagent A on ice for 15 min, then homogenised in an ice-cold glass homogeniser. After 50 strokes, the homogenates were centrifuged at 700 × g for 10 min at 4°C, and the supernatants were obtained without disturbing the pellet. The samples were centrifuged at 14 000 × g at 4°C for 30 min to pellet the cell membrane fragments. The supernatant contains cytoplasmic proteins. A total of 30–50 µL of supernatant was left to avoid contamination with the pellet. The supernatants containing membrane proteins were centrifuged at 14 000 × g for 10 s. After vortexing and incubation 1–2 times to extract membrane proteins thoroughly, the supernatants were centrifuged at 14 000 × g for 5 min, and the supernatants containing membrane proteins were then obtained.
2.28 Lysotracker DND-99 staining of lysosomes
HUVECs were cultured on six-well chambered coverslips, treated accordingly, and incubated with 1 µM Lysotracker DND-99 (Invitrogen, L7528) for 10 min at 37°C. After three washes with fresh medium, the cells were analysed by fluorescence microscopy.
2.29 LTR uptake in HUVECs
The treated HUVECs were incubated with a LTR fluorescent probe (50 nM, Invitrogen, L12492) for 2 h at 37°C and the excess dye was washed. Then, the cells were examined and imaged using a microscope (Olympus, Japan).
2.30 Lysosome isolation
Lysosomal fractions from tissues and cells were enriched and purified using the Lysosome Isolation Kit (Abcam, ab234047) by differential centrifugation followed by density gradient centrifugation.46, 47 Briefly, samples were homogenised in ice-cold lysosome isolation buffer for 2 min. The supernatants were collected by centrifugation at 500 × g and layered onto a discontinuous density gradient. Lysosomes were then isolated using ultracentrifugation at 145 000 × g for 2 h, after which the lysosomal fraction was obtained for further analysis.
2.31 Experimental design
To explore the roles of NKAα1 in diabetes-related endothelial dysfunction, this study investigated the effects of high-glucose (HG) exposure on mouse aortae and ECs as well as HFD-induced diabetes in WT and NKAα1+/− mice. Protein and mRNA levels of NKAα1 were measured and NKA activity was also evaluated in these tissues and cells to determine the impact of diabetic conditions on NKAα1 function. EDR was tested in aortic rings from WT, NKAα1+/−, and NKAα1-overexpressing mice. SNP was used to evaluate endothelium-independent relaxation.
Oxidative/nitrative stress markers, including superoxide, NOx, and nitrotyrosine, were measured in HG/HFD-induced diabetic mouse aortae and ECs. EC viability, proliferation, migration, and tube formation were measured following NKAα1 overexpression or knockdown. The effects of NKAα1 on EC apoptosis were assessed through TUNEL assays.
To investigate the role of NKAα1 in ferroptosis, ferroptosis markers, including Fe2⁺ levels (FerroOrange staining), GSH levels, PTGS2 mRNA, Fth1 mRNA, ACSL4, and GPX4 protein levels, were measured. Autophagy-related protein expression, including LC3II/LC3I ratio and p62, was examined in HG/HFD-exposed ECs and mouse aortae. The localisation of ACSL4 within lysosomes was analysed to assess lysosomal degradation efficiency. LMP was detected via double immunostaining with LGALS3 and LAMP1, and lysosomal pH changes were assessed with LysoTracker Red staining.
Eventually, a small library of 379 natural compounds was screened to identify potential NKAα1 activators. Hamaudol was identified and examined for its effects on NKAα1 expression, activity, and translocation in diabetic mouse aortae. After 6 weeks of normal diet (ND) or HFD, mice were subjected to intraperitoneal injection of Hamaudol (30 mg/kg) or sterile saline for the next 6 weeks under ND or HFD conditions.48 HUVECs were preincubated with Hamaudol (5 µM) for 30 min and were incubated with HG/HF for addition 24 h. The effects of Hamaudol on EDR, ferroptosis markers, oxidative/nitrative stress, ACSL4 and Cathepsin B expression, and autophagy were assessed to elucidate its therapeutic potential.
2.32 Statistical analysis
Results are presented as mean ± standard deviation (SD). In vitro experiments included at least three or four independent biological and technical replicates, while in vivo experiments had six replicates. Data analysis and graphing were performed using GraphPad Prism. Differences between two groups were assessed using an unpaired t-test, and multiple treatment comparisons were analysed with ANOVA followed by the Bonferroni post hoc test. A p-value < .05 was considered statistically significant.
3 RESULTS
3.1 Role of NKAα1 in diabetes-elicited endothelial dysfunction
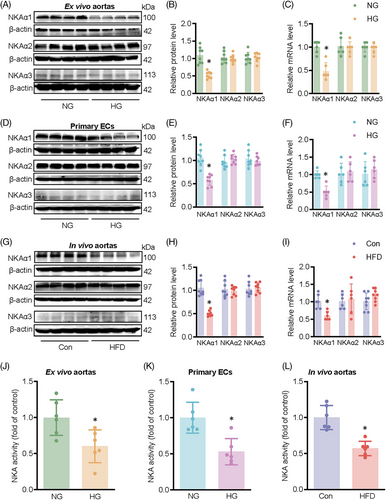
HG exposure obviously reduced the protein and mRNA expression levels of NKAα1 (Figure 1A–C), accompanied by a decreased NKA activity in mouse aortae (Figure 1J). To detect the expression and distribution of NKAα1 in human aortae, we used the online database HUMAN PROTEIN ATLAS (https://www.proteinatlas.org/) to compare the differences in NKAα1 expression levels in human aortae. It is shown that NKAα1 is mainly expressed in ECs of human aortae (Figure S1A and B). Primary cell experiments showed that NKA1 was mainly expressed in ECs, and high glucose did not affect the expression of NKA1 in vascular smooth muscle cells and vascular fibroblasts (Figure S1C). Thus, we focused on studying the role of NKAα1 in diabetes-elicited endothelial dysfunction. Exposure to HG resulted in a marked decrease in both protein and mRNA levels of NKAα1 in ECs (Figure 1D–F), as well as disrupted NKA activity (Figure 1K). These changes were also detectable in HFD-fed mice (Figure 1G–I and L). Interestingly, the translational and transcriptional levels of NKAα2 and NKAα3 remain unchanged in HG-challenged aortae and ECs, as well as in aortae from HFD mice (Figure 1A–I). Overall, these results indicate that NKAα1 expression and activity are significantly impaired in endothelial dysfunction associated with diabetes.
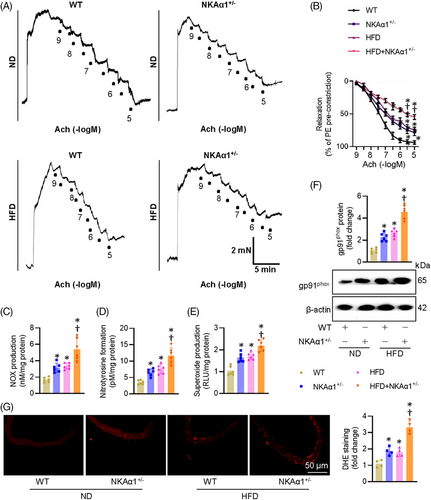
The aortic rings of heterozygous NKAα1+/− mice exhibited reduced endothelium-dependent relaxation (EDR) in comparison with those in wild-type (WT) mice (Figures 2A and S2A). Diabetic aortic segments showed higher levels of NOx, nitrotyrosine, and superoxide anions, this was further aggravated in NKAα1+/− mice fed by a HFD (Figure 2B–D). Immunoblotting for gp91phox and DHE staining showed increased oxidative stress in both NKAα1+/− mice and diabetic mice, with these changes becoming more pronounced in HFD-fed NKAα1+/− mice (Figure 2E–G). Conversely, EC-specific overexpression of NKAα1 via adeno-associated virus (AAV) ameliorated EDR in HFD mice by suppressing oxidative and nitrative stresses (Figure S3A–G). Intriguingly, the addition of the NO donor sodium nitroprusside (SNP), resulted in full vascular relaxation in all groups (Figure S4A and B). We then moved to assess the potential role of NKAα1 in EDR of ex vivo aortae incubated by HG. In line with in vivo results, EDR in HG-exposed aortae was obviously reduced, this was further declined in mouse aortae from NKAα1 knockdown mice (Figure S5A). SNP-induced vascular relaxation responses were not altered by loss of NKAα1 (Figure S5B). In addition, the production of NOx, nitrotyrosine, and superoxide anions tended to be higher in HG-exposed ex vivo aortae, an effect that was further amplified in NKAα1-deficient aortae (Figure S5C–E). By contrast, overexpression of endothelial NKAα1 by AAV5-TIE1-NKAα1 reversed these effects (Figure S5F–J). HFD mice showed higher body weight, increased levels of fasting blood glucose (FBG), upregulated serum levels of insulin, total cholesterol, and triglycerides compared to control mice (Tables S3 and S4).
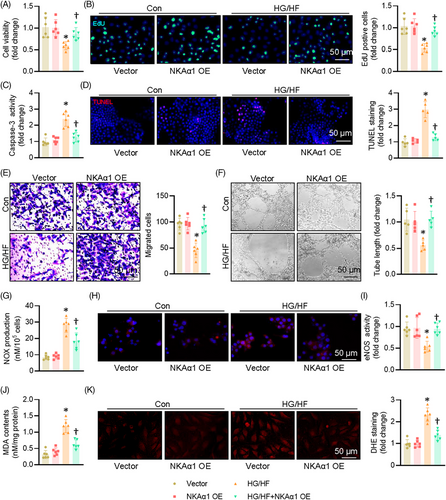
3.2 Role of NKAα1 in HG/HF-elicited endothelial cell injury
Compared with the normal glucose (NG) group, the cell viability of ECs was obviously decreased after HG/HF exposure, an effect that was partially reversed by overexpression of NKAα1 (Figures 2A and S6A). Overexpression of NKAα1 increased the proportion of EdU-positive cells in the context of HG/HF (Figure 3B). HG/HF caused an obvious increase in caspase 3 activity, which was attenuated by NKAα1 overexpression (Figure 3C). The TUNEL-positive cells were higher in HG/HF-incubated cells, whereas NKAα1 overexpression reversed this phenomenon (Figure 3D). Upregulation of NKAα1 led to increased number of migrated ECs under HG/HF conditions (Figure 3E). Additionally, NKAα1 overexpression significantly abrogated HG/HF-impaired tube formation (Figure 3F). HG/HF caused the overproduction of NOx (Figure 3G) and nitrotyrosine (Figure 3H), while inhibiting the activity of eNOS in ECs (Figure 3I), effects that were mitigated by NKAα1 overexpression. Excessive formation of MDA and superoxide anions were observed in ECs upon HG/HF exposure, and these changes were reversed by NKAα1 overexpression (Figure 3J and K). Paralleling, the protein levels of IL-1β, MCP-1, TNF-α, and VCAM-1 were significantly enhanced in HG/HF-exposed ECs, and NKAα1 overexpression eliminated these changes (Figure S6B). Similar results were also observed by real-time PCR assays (Figure S6C). On the contrary, silencing NKAα1 further worsened the detrimental actions of HG/HF on cell viability, apoptosis, migration, tube formation, oxidative stress, and inflammation in ECs (Figures S6D–F and S7).
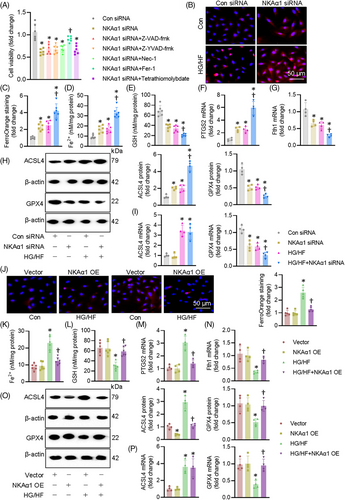
3.3 Impaired NKAα1 function results in EC ferroptosis in vitro and in vivo
We then examined how NKAα1 malfunction contributed to EC injury induced by HG/HF. The suppressive effects of NKAα1 knockdown on the cell viability were significantly mitigated by treatment a ferroptosis inhibitor Fer-1, but not by Z-YVAD-fmk, Z-VAD-fmk, Nec-1, or Tetrathiomolybdate, which are well-known inhibitors of apoptosis, pyroptosis, necrosis, and cuproptosis, respectively (Figure 4A). This indicates that NKAα1 impairment may be related to ferroptosis in ECs. Therefore, ferroptosis-related indicators were tested in ECs. As anticipated, HG/HF induced overproduction of Fe2+, as indicated by FerroOrange staining and Fe2+ levels in ECs, such effects were aggravated by knocking down NKAα1 (Figure 4B–D). Knockdown of NKAα1 exacerbated HG/HF-induced decreases in the contents of GSH (Figure 4E). Supportively, HG/HF stimulation elevated the mRNA level of PTGS2 and suppressed the transcriptional level of ferritin heavy chain1 (Fth1) in ECs, an observation that was intensified by silencing NKAα1 (Figure 4F and G). Western blot analysis showed that NKAα1 knockdown further disrupted the actions of HG/HF on the protein expression levels of ACSL4 (a proferroptosis protein) and GPX4 (an antiferroptosis protein) (Figures 4H and S8A). NKAα1 knockdown significantly enhanced the HG/HF-induced downregulation of GPX4 mRNA, but not ACSL4 mRNA (Figure 4I). Although NKAα1 overexpression (Figure S8B) did not affect the mRNA level of ACSL4, it is sufficient to attenuate HG/HF-induced ferroptosis in ECs (Figure 4J–O). Overexpression of NKAα1 reduced the levels of long-chain PUFA-containing phospholipids, as manifested by targeted lipidomic analysis (Figure S9A). In support, Erastin, a known ferroptosis inducer, abolished the protective effects of NKAα1 overexpression on HG/HF-induced apoptosis, oxidative/nitrative stresses, rather than inflammatory response in ECs (Figure S10A–D). Taken together, we found that decreased NKAα1 activity exacerbated HG/HF-induced ferroptosis, indicating a central role of NKAα1 in the ferroptosis induced by HG/HF.
In consistence with cellular results, NKAα1-deficient mice were predisposed to ferroptosis in aortae after HFD feeding (Figures 5A–H and S8C), while endothelial specific overexpression of NKAα1 increased the resistance of mouse aortae to HFD-induced ferroptosis (Figures 5I–O and S8D). Similar results were obtained from primary ECs in WT and NKAα1 heterozygous mice (Figure S11A–D). To further confirm the fundamental role of ferroptosis in NKAα1 deletion in vascular endothelial dysfunction in diabetes, we used Fer-1 and Erastin in mouse aortae. As expected, administration of a ferroptosis inhibitor Fer-1 restored impaired EDR in aortae from NKAα1+/− mice (Figure S12A), in conjunction with decreased contents of superoxide, NOx, and nitrotyrosine in aortic tissues (Figure S12B–D). Meanwhile, the benefits of NKAα1 overexpression in EDR were eliminated by the presence of Erastin, an inducer of ferroptosis (Figure S12E). Erastin treatment prevented the suppressive actions of NKAα1 overexpression on oxidative/nitrative stresses in aortae from HFD-fed mice (Figure S12F–H).
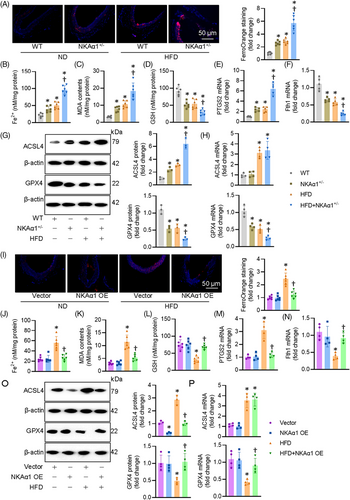
3.4 Role of NKAα1 in diabetes-triggered impairment in lysosome biosynthesis and function
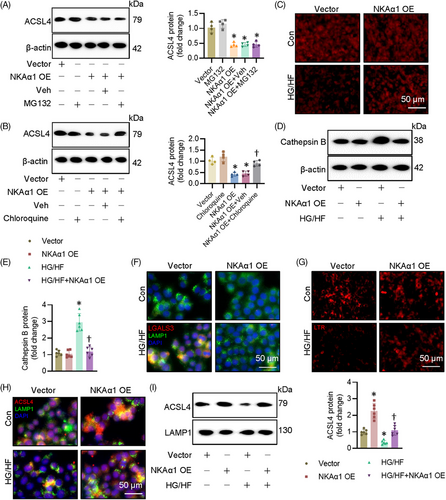
NKAα1 inversely affected the protein expression of ACSL4 without influencing its mRNA level, indicating the posttranslational modification might mediate the actions of NKAα1 on ACSL4. It is well established that the uniquitin-proteasome system and the lysosome system can mediate the degradation of intercellular protein. To determine which pathway is responsible for NKAα1-induced ACSL4 degradation, we treated ECs with proteasome and lysosome inhibitors. NKAα1-induced downregulation of ACSL4 was significantly attenuated by chloroquine, a lysosome inhibitor, but not by a proteasome inhibitor MG132 (Figure 6A and B). This suggests that the lysosome system may be involved in NKAα1 knockdown-induced upregulation of ACSL4. As depicted in Figure 6C, HG/HF markedly decreased lysotracker staining, but this was prevented by NKAα1 overexpression. We also observed that NKAα1 overexpression reduced cathepsin B accumulation in ECs upon HG/HF exposure (Figure 6D and E), suggesting that NKAα1 overexpression is able to prevent the release of cathepsin B from lysosomes. The lysosome membrane permeabilisation (LMP) is reported to contribute to impaired autophagy degradation. To investigate whether LMP occurs in ECs following HG/HF exposure, double immunostaining for LGALS3 and LAMP1 was performed. The results showed an increase in LGALS3-positive dots in HG/HF-exposed cells, with a marked colocalisation of LGALS3 and LAMP1 puncta. These changes were significantly reversed by NKAα1 overexpression (Figure 6F). To further assess LMP, lysosomal pH was evaluated using the LysoTracker Red (LTR) fluorescent probe, which selectively labels acidic organelles in live cells. LTR fluorescence was reduced in ECs after HG/HF exposure, but this effect was reversed upon NKAα1 overexpression (Figure 6G). These findings suggest that LMP may contribute to the reduced autophagic degradative capacity in ECs under HG/HF conditions, a dysfunction that can be corrected by NKAα1 overexpression. To corroborate such observations, we measured the protein expression of ACSL4 in the lysosome. Both immunostaining and immunoblotting showed that NKAα1 overexpression prevented the effects of HG/HF on the location of ACSL4 in the lysosome (Figure 6H and I). Inversely, silencing NKAα1 further decreased the accumulation of ACSL4 in the lysosome of ECs challenged by HG/HF (Figure S13A). The lysosome inhibitor chloroquine prevented the actions of NKAα1 overexpression on cell apoptosis, oxidative/nitrative stresses, and ferroptosis in ECs after HG/HF challenge (Figure S14A–G). The antagonistic effects of NKAα1 overexpression on oxidative/nitrative stresses and EDR impairment were also impeded after administration of chloroquine (Figure S14H–M).
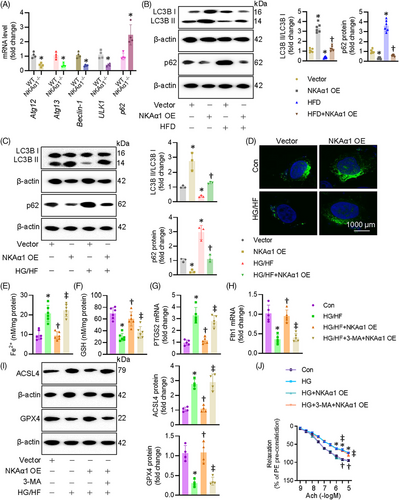
3.5 NKAα1 deficiency led to autophagy dysfunction in diabetes-induced endothelial injury
Autophagy is a cleansing and reparative process that delivers cellular cargoes to lysosomes for degradation. This process begins with the formation of autophagosomes, requiring class III phosphoinositide 3-kinase (PI3K) Vps34 to interact with beclin-1. After membrane elongation, autophagosomes fuse with lysosomes, allowing lysosomal hydrolases to degrade the waste in cells. We previously demonstrated that NKAα1 deficiency resulted in the disruption of autophagy, a critical event in nonalcoholic fatty liver disease, brain ischemic injury, and Parkinson's disease. Based on this, we hypothesised that malfunction of NKAα1 might cause the disordered autophagy-lysosome pathway in diabetic endothelial dysfunction. As shown in Figure 7A, the mRNA levels of autophagy-related genes, including Atg12, Atg13, beclin-1, ULK1 and p62, were significantly altered in mouse aortae after HFD feeding. Immunoblotting results showed that the aortae from HFD mice exhibited lower expression of LC3II/LC3I ratio and higher expression of p62, both of which were reversed by endothelial-specific overexpression of NKAα1 (Figure 7B). The mRNA level of beclin-1 was lower, while the transcriptional level of p62 was higher in isolated ECs from NKAα1 heterozygous mice than those from WT mice (Figure S11E and F). In a similar way, overexpression of NKAα1 restored LC3II/LC3I ratio and downregulated p62 protein expression in ECs exposed to HG/HF (Figure 7C). In line with this, NKAα1 overexpression increased the number of LC3 puncta-positive cells and reversed the reduction in LC3 puncta observed in HG/HF-treated ECs (Figure 7D). Blockaded of autophagy by 3-MA abolished the suppressive actions of NKAα1 overexpression on ferroptosis (Figure 7E–I) and cell injury induced by HG/HF (Figure S15A–D). Most importantly, the vasodilation effects of NKAα1 overexpression were significantly weakened when the autophagy was inhibited by 3-MA (Figure 7J). In compliance with these findings, 3-MA pretreatment overtly abolished the protective effects of NKAα1 on oxidative/nitrative stresses in HG-incubated mouse aortae (Figure S16A–D).
NKAα1 has been shown to interact with specific cell signalling pathways, including the mTOR and AMPK pathways, both of which play key roles in regulating autophagy.17, 49 Thus, we examined whether NKAα1 induced autophagy processes in ECs by regulating the AMPK/mTOR pathway. Consistent with our previous studies,17, 49 we found that NKAα1 interacted with AMPKα1, and this complex was higher in HG-induced ECs (Figure S17A). NKAα1 overexpression induced AMPK phosphorylation, attenuated the increased phosphorylation of mTOR (p-mTOR; Ser2448) and reversed the decreased phosphorylation of ULK1 (p-ULK1, Ser555) and AMPK (p-AMPK, Thr172) in HG-challenged ECs (Figure S17B). These findings suggest that NKAα1/AMPKα1 interaction could exert a regulatory effect on the autophagy process in ECs, likely through the AMPK/mTOR/ULK1 signalling pathway.
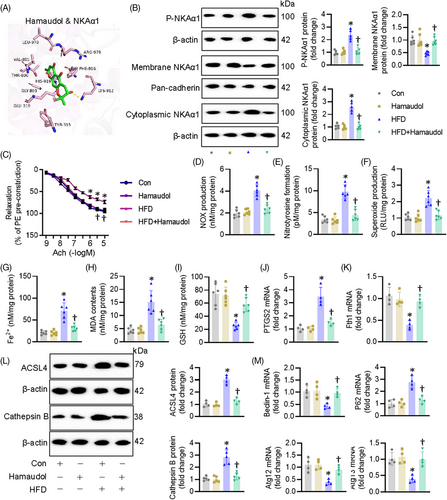
3.6 Hamaudol is identified as an activator of NKAα1 with a protection against diabetes-induced endothelial dysfunction
Based on the data above, overexpression of NKAα1 appears to be a feasible therapeutic strategy for diabetes-evoked endothelial dysfunction. For this reason, we sought to screen a potential activator of NKAα1 from a small library containing 379 natural products. Our screening results showed that Hamaudol displayed the strongest abilities to enhance NKAα1 activity (Table S5). Molecular docking results confirmed the direct direction interaction between Hamaudol and NKAα1, with a binding energy of −5.64 KJ/mol (Figure S18A). More specifically, 10 hydrogen bonds were formed between Hamaudol and the Tyr315, Glu319, Gly803, Val805, Thr806, Lys912, Phe916, His919, Leu978, and Arg979 in the ligand-binding domain of NKAα1 (Figure 8A). Functional results demonstrated that pretreatment with Hamaudol restored the protein expression of NKAα1 and NKA activity in ECs exposed to HG/HF (Figure S18B and C). Cellular experiments showed that Hamaudol increased EC viability, promoted the migration and tube formation of ECs under HG/HF stress (Figure S18D–G). Impaired activity of NKA is found to be its phosphorylation and cytoplasmic translocation, and this event leads to a variety of oxidative stress-related diseases. To this end, we measured the phosphorylation levels of NKAα1 and the membrane and cytoplasmic abundance of NKAα1. Hamaudol treatment reduced NKAα1 phosphorylation and prevented its translocation from the membrane to the cytoplasm in HFD-induced mouse aortae (Figure 8B), accompanied by restored NKA activity (Figure 8C). Furthermore, Hamaudol treatment diminished diabetes-induced EDR impairment (Figure 8D). The contents of NOx, nitrotyrosine, and superoxide anions in diabetic mouse aortae were obviously downregulated by treatment with Hamaudol (Figure 8E–G). The Fe2+ contents and lipid peroxidation levels were markedly raised in diabetes and significantly reduced after treatment with Hamaudol (Figure 8H and I). In addition, Hamaudol supplementation retarded ferroptosis, as evidenced by GSH levels, mRNA levels of PTGS2 and Fth1 altered by the HFD (Figure 8J and K). In accordance with the results of NKAα1 overexpression, administration of Hamaudol prevented diabetes-induced protein upregulations of ACSL4 and Cathepsin B (Figure 8L), and induced autophagy in diabetic mouse aortae (Figure 8M). Interestingly, administration of Hamaudol had no effect on the fasting blood glucose and lipid metabolism in HFD-induced mice (Table S6). Overall, these findings suggest that Hamaudol achieved its favourable effects in the context of diabetes-evoked endothelial cell dysfunction by targeting NKAα1 and inhibiting ferroptosis. Vascular remodelling is a well-established pathological feature in diabetes, often characterised by endothelial dysfunction, vascular smooth muscle cell proliferation, increased collagen deposition, and thickening of the arterial walls. It is interesting to know whether Hamaudol has an effect on vascular remodelling in diabetic mice. Results showed that the lumen diameter was significantly downregulated, along with a marked increase in medial thickness and the medial thickness/lumen diameter ratio in diabetic mice in comparison with the control mice. These alterations were attenuated by administration of Hamaudol (Figure S19A–D). The mRNA levels of pro-proliferative, propro-fibrotic and proinflammatory genes were substantially upregulated in the aortae of HFD mice, which were largely downregulated by Hamaudol treatment (Figure S19E–G). These results indicate that Hamaudol not only mitigates endothelial dysfunction but also helps alleviate vascular remodelling processes in the context of diabetes.
4 DISCUSSION
The present study demonstrates an important role for NKAα1 in improving diabetes-related endothelial dysfunction induced by a HFD diet. In vitro, ex vivo and in vivo research disclosed lower NKA activity and NKAα1 expression in diabetic endothelial dysfunction. We found that NKAα1 haploinsufficiency significantly aggravated diabetes-elicited endothelial dysfunction in mice and silencing NKAα1 worsened HG/HF-induced impairment in EC viability, migration and angiogenesis. Endothelial cell-specific overexpression of NKAα1 protected against such dysfunction in diabetes. Mechanistically, NKAα1 deficiency impaired the autophagy-lysosome pathway, thereby leading to reduced ACSL4 degradation and enhanced lipid peroxidation and ferroptosis (Figure 9). Inhibition of the autophagy-lysosome pathway blocked the protection of NKAα1 overexpression against endothelial damage in diabetes. Most importantly, from a small natural product compound library, we identified Hamaudol as a NKAα1 activator by reducing the phosphorylation and endocytosis of NKAα1, and this compound displayed similar vasoprotective benefits as overexpression of NKAα1 in vivo and in vitro. With the aid of gain-of-function and loss-of-function approaches, we demonstrated that targeting NKAα1 may be recommended as a promising avenue for the management of vascular complications associated with diabetes.
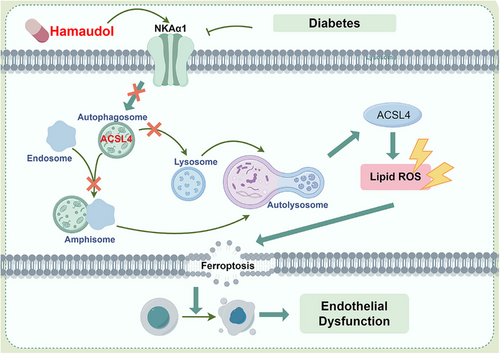
In this study, our results demonstrate that deficiency or dysfunction of NKAα1 compromises vasomotor function in the aortae of HG-exposed and HFD-induced diabetic mice, reflected by a significant reduction in EDR responses. These effects were aggravated by NKAα1 knockdown but ameliorated by its overexpression, suggesting that NKAα1 preservation is crucial for maintaining vasomotor tone in diabetic contexts. Given that impaired vasodilation is a hallmark of endothelial dysfunction in diabetes, our findings position NKAα1 as a potential target to restore vasomotor function. This aligns with previous reports linking NKAα1 dysfunction to cardiovascular and metabolic diseases, although our study uniquely emphasises its critical role in diabetic vasculature and suggests that enhancing NKAα1 activity could counter diabetes-related vascular complications. The higher expression of NKAα1 in ECs could reflect the specialised role of NKAα1 in maintaining ion gradients and membrane potential within the endothelium, which is crucial for regulating endothelial cell function, vascular tone, and permeability. The aorta, being a complex tissue with multiple cell types, may show a more diluted or lower level of NKAα1 expression when averaged across all cell types. One should bear in mind that other subunits of NKA may play different roles in different types of vascular cells, which deserves further exploration in the future.
Our study extends beyond the vasodilatory role of NKAα1 by showing that NKAα1 directly supports EC viability, migration, and angiogenesis, fundamental processes for vascular repair and integrity. The observed improvements in EC function following NKAα1 overexpression and the detrimental effects of NKAα1 knockdown suggest that NKAα1 actively participates in maintaining cellular health. Mechanistically, we found that NKAα1 suppressed oxidative and nitrative stress markers, including superoxide, NOx, and nitrotyrosine, in HG/HF-challenged ECs. This indicates that NKAα1 mitigates oxidative damage by possibly modulating ion gradients and membrane stability, which are critical to reduce ROS formation. These results emphasise a critical role of NKAα1 as a cellular protector against oxidative stress, a major driver of endothelial dysfunction in diabetes.
It was recently reported that impaired NKA activity is involved in the pathological process of doxorubicin-induced cardiotoxicity14 and Parkinson's disease .16 We therefore further moved on to assess whether NKAα1 protected against diabetes-induced endothelial dysfunction by suppressing ferroptosis. In this study, we found that overexpression of NKAα1 was sufficient to suppress HG/HF-induced ferroptosis in ECs, while the development of ferroptosis was further aggravated in NKAα1-dificient ECs exposed to HG/HF. Similar results were replicated in HFD mice. Our present results indicated that NKAα1 overexpression profoundly dampened diabetes-provoked endothelial injury by suppressing ferroptosis. Under diabetic conditions, the increase in Fe2⁺ levels can be attributed to enhanced oxidative stress and lipid peroxidation, both of which are known to alter cellular iron homeostasis. Excessive iron in its ferrous form (Fe2⁺) can generate ROS through the Fenton reaction, exacerbating oxidative stress in cells exposed to diabetes. This environment often disrupts iron regulatory proteins and may increase cellular iron uptake or decrease iron storage, leading to elevated Fe2⁺ levels in cells. When NKAα1 is overexpressed in ECs exposed to diabetes, it likely mitigates these effects by reducing oxidative stress and stabilising iron homeostasis. NKAα1 is known to maintain cellular ion gradients and modulate intracellular signalling pathways related to oxidative stress responses. By decreasing ROS levels, NKAα1 overexpression could reduce Fe2⁺ accumulation, as less ROS production can decrease the dysregulation of iron metabolism. Moreover, NKAα1 may help to regulate the expression of iron transporters and storage proteins, leading to a decrease in intracellular Fe2⁺ levels. In summary, the increased Fe2⁺ levels under diabetic conditions were likely resulted from oxidative stress-induced iron dysregulation. NKAα1 overexpression helps to reverse this effect by alleviating oxidative stress and restoring iron homeostasis, thus lowering Fe2⁺ levels in this context.
A plethora of proteins are postulated to contribute to the process of ferroptosis, such as antiferroptosis genes, such as SLC7A11 and GPX4, and proferroptosis genes, including PTGS2, TFRC, and ACSL4.50 Among them, ACSL4 has gained a widespread recognition in the development of ferroptosis since ACSL4 is able to shape cellular lipid composition by increasing the contents of long polyunsaturated ω6 fatty acids, a central event in ferroptosis.51, 52 ACSL4-mediated regulation of ferroptosis is involved in high-fat-diet (HFD)-induced cardiac remodelling and dysfunction.53 In this study, we found that NKAα1 overexpression enormously obliterated the protein expression of ASCL4 and the contents of long-chain PUFA-containing phospholipids species in ECs exposed to HG/HF. Neither NKAα1 overexpression nor NKAα1 downregulation affected the mRNA of ACSL4, indicating that NKAα1 regulated the expression ACSL4 in a manner that was dependent on the protein posttranslational modification mechanism. Further studies revealed that chloroquine, a lysosome inhibitor, prevented NKAα1-induced downregulation of ACSL4, indicating that the disrupted lysosome system may participate in NKAα1 deficiency-induced upregulation of ACSL4. This novel insight highlights a protective feedback mechanism where NKAα1 not only mitigates oxidative stress but also facilitates cellular clearance processes that prevent ferroptosis in diabetes. By preserving lysosomal function, NKAα1 may also prevent the release of iron from lysosomes, thus further reducing ferroptosis risk, a finding that opens new therapeutic avenues targeting lysosomal and autophagic processes in diabetes.
In our study, we observed that NKAα1 overexpression appears to stabilise lysosomes, potentially inhibiting LMP and thereby preserving lysosomal integrity. The prevention of LMP would likely reduce the release of iron from lysosomes into the cytoplasm. This stabilisation could indeed protect cells under HG stress, not only by reducing free cytosolic iron that would otherwise catalyse ROS production and promote ferroptosis but also by maintaining cellular iron homeostasis. Regarding ferritin, if LMP is blocked by NKAα1 overexpression, ferritin within lysosomes would remain stable, as it would not be subjected to lysosomal degradation. This could explain why ferritin levels are maintained, reducing the availability of free iron and thereby limiting iron-catalysed lipid peroxidation, a key feature of ferroptosis. Thus, NKAα1 overexpression may indirectly contribute to ferroptosis inhibition by restricting iron efflux rather than only directly influencing ACSL4 degradation alone. In this context, ACSL4, an enzyme involved in synthesising proferroptotic lipids, would be less impactful if iron levels are controlled, as the substrate for lipid peroxidation would be limited. This suggests that the protective mechanism of NKAα1 might be related to reducing iron release via lysosomal stabilisation and subsequently limiting conditions conducive to ferroptosis. In summary, while our study originally focused on ACSL4 degradation as a marker for autophagic impairment, it is likely the protective role of NKAα1 may also involve the prevention of iron efflux due to stabilised lysosomes, thereby indirectly reducing ferroptosis, which needs more solid evidence.
By screening our phytochemical compound library, we found that the phytochemical Hamaudol directly binds to NKAα1 and induces its activation by suppressing the phosphorylation and endocytosis of NKAα1. Our animal and cellular results consistently disclosed that Hamaudol treatment significantly improved endothelial dysfunction under diabetic state by restoring NKAα1 protein expression and inhibiting ACSL4-mediated ferroptosis. Interestingly, the effects of Hamaudol were specific to the functional enhancement of NKAα1, as it did not significantly alter glucose or lipid profiles in HFD mice. This specificity may allow Hamaudol to offer vascular benefits in diabetes without affecting broader metabolic parameters, making it a focused therapeutic candidate. Given the involvement in both autophagy and ferroptosis regulation of NKAα1, activating NKAα1 with compounds like Hamaudol could provide a novel approach to prevent ferroptosis and oxidative damage in diabetic vasculature. Although the benefit of Hamaudol in diabetic vascular remodelling, more studies are recommended to perform specific experiments in diabetic mouse models to evaluate vascular remodelling and cardiovascular events following Hamaudol treatment.
5 CONCLUSIONS
In summary, our characterisation of the autophagy-lysosome pathway dysregulation and subsequent ferroptosis provides novel mechanistic insights into the potential role of NKAα1 deficiency in the development of endothelial cell dysfunction induced by diabetes. Our study also revealed that the phytochemical Hamaudol activated NKAα1 to ameliorate endothelial damage, making it a promising drug candidate for managing diabetes-associated cardiovascular ailments.
Although our study provides strong evidence for the role of NKAα1 in endothelial dysfunction under diabetic conditions, several limitations should be presented in the study. Firstly, although our in vivo findings in HFD-induced diabetic mice support solid evidence for the protective role of NKAα1 in vascular function, the results are derived from a single animal model of diabetes. It would be important to confirm these findings in other models of diabetes, such as genetically modified mice or nonobese diabetic (NOD) mice. Secondly, while our study focuses on ECs and their function, NKAα1 is also expressed in cardiomyocytes, smooth muscle cells, and its systemic effects on these tissues were not explored. Future studies could investigate whether the observed vascular benefits are solely due to endothelial NKAα1 modulation or if other cell types contribute to the overall therapeutic effects. Thirdly, future studies should investigate the potential of NKAα1 activators in clinical settings and investigate whether NKAα1 modulation could benefit other complications associated with diabetes, such as cardiomyopathy, neuropathy and nephropathy, where ferroptosis and oxidative stress are also implicated. Fourthly, NKAα1 heterozygous mice do not represent an EC-specific knockdown model. Future studies utilising EC-specific knockdown models (Cre-flox technology) are needed to further dissect the role of NKAα1 specifically in ECs under diabetic context. Fifthly, in our current study, ECs were not isolated from NKAα1 heterozygous and WT mice for a direct, cell-specific analysis of the pathways. While this approach provides valuable information about the systemic effects of NKAα1 on vascular tissues, isolating ECs would allow for more precise analysis of the pathways specifically within ECs. Future studies are required to isolate ECs from NKAα1 heterozygous and WT mice to confirm the observed functional changes at the cellular level. To refine our understanding of the specific pathways influenced by NKAα1 in endothelial cells, we plan to perform endothelial cell-specific analyses using primary EC cultures from NKAα1 heterozygous and WT mice. This will allow us to confirm whether apoptosis, ferroptosis, lysosome biosynthesis, and autophagy are indeed modulated by NKAα1 specifically in endothelial cells. Additionally, further research is needed to understand the precise signalling pathways that link NKAα1 to autophagy and lysosomal function in diverse types of vascular cells.
AUTHOR CONTRIBUTIONS
Xue-Xue Zhu, ZJH, Na Li, Hai-Jian Sun, and Qing-Bo Lu designed the experiment plans, wrote and reviewed the manuscript. Xue-Xue Zhu, Xin-Yu Meng, Fang-Ming Wang, Xiao-Ying Chai, Guo Chen, An-Jing Xu, Jia-Bao Su, and Hong-Bo Qiu carried out the experiments and data analyses. Qing-Yi Sun, Zhi-Jun Han, Yao Wang, Zhuo-Lin Lv, Yao Liu, and Yuan Zhang provided technical support and intelligence. Hai-Jian Sun and Qing-Bo Lu served as the lead contact for this work.
ACKNOWLEDGEMENTS
This work was supported by the National Natural Science Foundation of China (82370364, 82274071, 82300414, and 82170424), Jiangsu Province Excellent Youth Foundation (BK20240204 ), Jiangsu Natural Science Foundation (BK20231049, BE2020634 and BK20191138), Joint research project of the Chinese Society of Traditional Chinese Medicine, high-level introduction of talents and scientific research start-up funds of JNU (1286010241222100), the Fundamental Research Funds for the Central Universities (JUSRP124036), Medical Discipline Program of Wuxi Health Commission, the Science and Technology Projects of Wuxi City (M202207, BJ2023029), Clinical Research and Translational Medicine Research Program of Affiliated Hospital of Jiangnan University (LCYJ202306, LCYJ202226), Wuxi Science and Technology Development Fund Project “Light of the Taihu Lake” (K20221028), Wuxi Municipal Health Commission Youth Project (Q202226), Top Talent Support Program for young and middle-aged people of Wuxi Health Committee (HB2023045, BJ2023045, BJ2023029). Medical technology research project of Wuxi Science and Technology Bureau(Y20232017) and Wuxi Taihu Lake Talent Plan, Supports for Leading Talents in Medical and Health Profession.
CONFLICT OF INTEREST STATEMENT
The authors declare no competing interests.
ETHICS STATEMENT
All experiments in mice were reviewed and approved by Animal Care and Use Committee of the China Pharmaceutical University (202101017).
Open Research
DATA AVAILABILITY STATEMENT
Source data for all main figures and extended data figures are supplied with this paper. Experimental data supporting the plots within this paper and other findings of this study are available from the corresponding author upon reasonable request.




