Interferon regulatory factor 5 suppresses epithelial-to-mesenchymal transition and metastasis by inducing GATA2 expression in colorectal cancer
Dear Editor,
Approximately one-third of colorectal cancer (CRC) patients develop metastasis, resulting in a mere 15% five-year relative overall survival rate, underscoring metastasis as a pressing clinical concern. [1] Epithelial-to-mesenchymal transition (EMT) is a biological process that confers high plasticity to cells and is heavily implicated in cancer metastasis and progression. [2, 3] Interferon regulatory factor 5 (IRF5) can induce target gene expression by binding to their promoters upon translocation to the nucleus upon activation. [4] Despite its well-known role as a key mediator of immune regulation, IRF5 has been reported to regulate the migratory capacity of cancer cells through transcription-dependent/independent mechanisms. [5] We, here, investigated the role of IRF5 in CRC metastasis regulation.
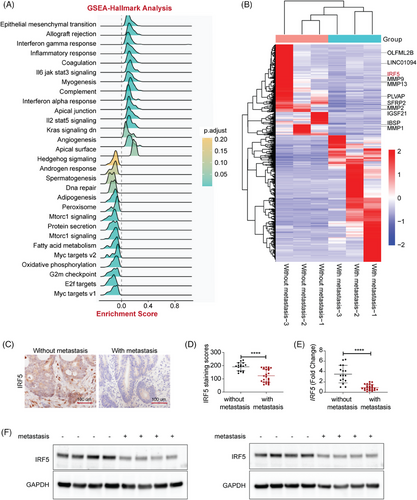
We first performed gene set enrichment analysis on normalized IRF5 expression data from the Cancer Genome Atlas (TCGA) database. IRF5 expression was strongly associated with EMT signalling (Figure 1A). Three pairs of primary colorectal tumour tissues and liver metastatic counterparts were collected for RNA-Seq analysis. Results showed significantly downregulated expression of IRF5 in metastatic tissues compared to primary ones, with other key regulators of metastasis showing up, including SFRP2, OLFML2B, PLVAP, IBSP, COL11A1, MMP, etc. (Figure 1B). Immunohistochemistry (IHC) analysis of tumour samples revealed significant downregulation of IRF5 in tissues with distant metastasis (Figure 1C,D). Real-time quantitative polymerase chain reaction (RT-qPCR) and western blot showed that IRF5 expression had a decrease in CRC tissues with metastasis compared to counterparts without metastasis, confirming decreased IRF5 expression at both transcriptional and translational levels (Figure 1E,F). Using the chi-square test, we found an inverse correlation between IRF5 and tumour metastasis, including N stage and M stage (Table S1).
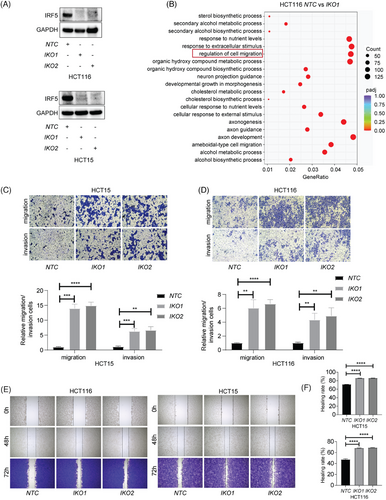
To test the involvement of immune regulation, multiple public datasets were analyzed to examine the relationship between IRF5 expression and diverse immune cell types. Unexpectedly, the results showed that IRF5 was found to be significantly positively correlated with M2 macrophages (Figure S1). However, M2 macrophages have been identified to facilitate tumour metastasis. [6] Thus, the role of IRF5 involved in metastasis was not dependent on immune regulation. Further, we knocked out IRF5 (IKO) in HCT116 and HCT15 cells by CRISPR-Cas9 (Figure 2A) and analyzed transcriptional profiles of control (NTC) and IKO cells using RNA sequencing, revealing that cell migration and EMT-related pathways were observed (Figure 2B, Figure S2 and Table S2). Functional assays also demonstrated increased invasion and migration capabilities in IKO groups compared to controls (Figure 2C–F). Hence, our data pointed out that IRF5-mediated inhibition of metastasis is not tumour-microenvironment-dependent, yet relies on its tumour-intrinsic role.
We next identified 84 differentially expressed genes (DEGs) associated with cell migration in HCT15 and 134 in HCT116, with 34 overlapping genes. Notably, GATA2 was identified as significantly upregulated (Figure 3A and Tables S3 and S4). Validation of GATA2 mRNA and protein levels showed substantial upregulation in IKO groups (Figure 3B-C and S3A,B). IHC staining, RT-qPCR and western blot analysis of tumour samples also indicated an increased GATA2 in metastatic tissues (Figure 3D,E and S3C,D). Moreover, IRF5 levels in patient tissues were negatively correlated with GATA2 levels (Figure 3F). Clinically, high GATA2 expression was positively correlated with advanced T stage, N stage, and M stage (Table S5).
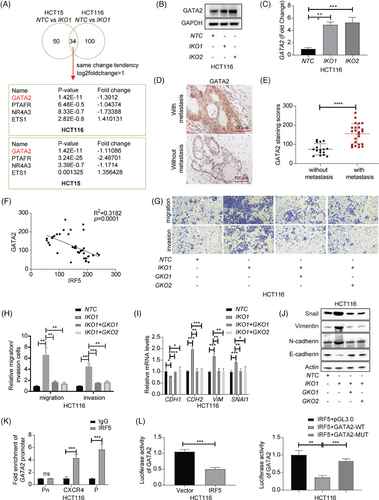
To investigate whether IRF5 inhibits metastasis and EMT process in CRC via repressing GATA2 induction, we established double knock-out cell models (DKO) by knocking out GATA2 (GKO) (Figure S3E). Deletion of GATA2 in IRF5-deficient cells significantly reduced their invasive and migratory capabilities (Figure 3G,H and Figure S3F–I). RT-qPCR and Western Blot analysis demonstrated a reversal in the expression of EMT-related markers upon GATA2 knockout (Figure 3I,J and S4A,B). Next, we wondered whether IRF5-mediated GATA2 suppression was transcriptionally-dependent. JASPAR database predictions showed that there might be a binding between IRF5 and -791 to -778 bp region of the GATA2 promoter (Figure S4C). Further, chromatin immunoprecipitation (ChIP) assays confirmed significant enrichment of the GATA2 promoter by anti-IRF5 antibody (Figure 3K and Figure S4D). Furthermore, a dual luciferase reporter assay demonstrated that IRF5 overexpression significantly decreased wild-type GATA2 promoter activity (Figure 3L and Figure S4E).
For further evaluation of IRF5's role in metastasis of CRC in vivo, we transplanted luciferase-labeled HCT15 NTC, IKO, and DKO cells into the spleens of nude mice. The IKO group showed increased luciferase signal intensities and reduced overall survival, both of which were mitigated by GATA2 knockout (Figure 4A–C). Additionally, the IKO group developed more metastatic nodules compared to the NTC or DKO groups (Figure 4D).
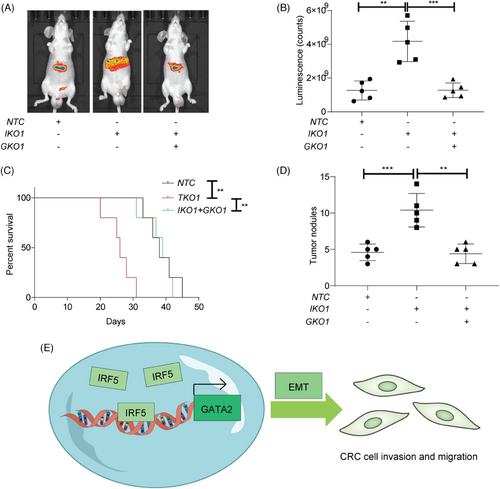
Metastatic CRC remains a formidable challenge with a dismal prognosis.[7] Therefore, it is urgent for the research community to find new targets to be specifically against metastasis for clinical application.[8] Genetic ablation of IRF5 showed that IRF5 affected the migration and invasion phenotype in CRC, highlighting that IRF5 contributed to tumour-intrinsic functions. Further RNA sequencing data uncovered GATA2 as the downstream target of IRF5. PCR and ChIP analyses proved that IRF5 transcriptionally regulated GATA2 expression. Notably, GATA2 is a potent driver of metastasis.[9, 10] By exploiting DKO cell models, our data further supported that GATA2 could mediate IRF5-induced suppression of EMT. Collectively, we found a novel mechanism by which IRF5 exerts tumour-intrinsic roles in regulating EMT machinery to inhibit metastasis (Figure 4E). However, further studies should be performed focusing on how GATA2 regulates EMT-associated targets. Given the multiple expression patterns and pleiotropic roles of IRF5, designing antagonists or agonists based on IRF5-GATA2 signalling will be the next step in the investigation for better clinical application.
AUTHOR CONTRIBUTIONS
Conception and design: Jinhai Deng, Xuerui Tan, Fengxiang Wei and Teng Pan. Writing the manuscript: Teng Pan and Jinhai Deng. Data analysis and performance of most of the experiments: Teng Pan. Data collection: Zaoqu Liu, Lifeng Li, Deyu Zhang, Qi Luo, Xiaojin Luo, Xiaohang Chen, Yao Yao, Guanglin Zhou, Jose M Vicencio, Weilong Zhang, Mingzhu Yin, Dang Wang and Richard Beatson.
ACKNOWLEDGEMENTS
Supported by the National Natural Science Fundation of China (No. 82403981), Shenzhen Science and Technology Program (No. JCYJ20220530162412029, No. JCYJ20240813144100002, No. JCYJ20210324111810028), Chongqing Natural Science Foundation (Grant No. CSTB2024NSCQ-MSX0761) and Medical and Health Science and Technology Plan attached to Longgang District Science and Technology Development Special Funding (LGKCYLWS2022008).
CONFLICT OF INTEREST STATEMENT
The authors declared no conflict of interest.
ETHICS STATEMENT
The study was authorized by the Institutional Ethics Committees of Chongqing University Three Gorges Hospital.
Open Research
DATA AVAILABILITY STATEMENT
Datasets are available from the corresponding author.




