COVID-19 critical illness pathophysiology driven by diffuse pulmonary thrombi and pulmonary endothelial dysfunction responsive to thrombolysis
Abstract
Patients with severe COVID-19 disease have been characterized as having the acute respiratory distress syndrome (ARDS). Critically ill COVID-19 patients have relatively well-preserved lung mechanics despite severe gas exchange abnormalities, a feature not consistent with classical ARDS but more consistent with pulmonary vascular disease. Many patients with severe COVID-19 also demonstrate markedly abnormal coagulation, with elevated d-dimers and higher rates of venous thromboembolism. We present four cases of patients with severe COVID-19 pneumonia with severe respiratory failure and shock, with evidence of markedly elevated dead-space ventilation who received tPA. All showed post treatment immediate improvements in gas exchange and/or hemodynamics. We suspect that severe COVID-19 pneumonia causes respiratory failure via pulmonary microthrombi and endothelial dysfunction. Treatment for COVID-19 pneumonia may warrant anticoagulation for milder cases and thrombolysis for more severe disease.
1 INTRODUCTION
Patients with severe COVID-19-induced respiratory failure demonstrate gas exchange abnormalities including shunt and dead-space ventilation. While patients with COVID-19 respiratory failure may fulfill the Berlin criteria for acute respiratory distress syndrome (ARDS), their syndrome is atypical in that the majority have relatively well-preserved lung mechanics.1 The marked dissociation between pulmonary mechanics and gas exchange raises the possibility of pulmonary vascular involvement.
We and others have observed a high rate of venous thromboembolism (VTE) in critically ill COVID-19 patients who otherwise lack the classic risk factors for VTE. d-Dimer levels have also been noted to be elevated, and rapid rises presage cardiopulmonary decompensation. A retrospective study from China demonstrated that the use of heparin was associated with improved mortality in patients with severe COVID-19 infection and significantly elevated d-dimers.2 An autopsy of a patient at our institution with severe COVID-19 disease revealed numerous pulmonary microthrombi.
We present four cases of COVID-19 patients, all between 55 and 65 years old, with refractory respiratory failure requiring mechanical ventilation and shock, who demonstrated evidence of elevated dead-space ventilation. We suspected significant pulmonary micro- and/or macrothromboses as drivers of this preterminal state and administered systemic tissue plasminogen activator (tPA). All cases had rapid improvement in alveolar ventilation, oxygenation, and/or shock.
We obtained consent from the legally authorized representatives for all patients. This study was reviewed by the Mount Sinai Institutional Review Board and was deemed exempt.
2 CASE 1
A 55-year-old woman with a history of obesity and diabetes with COVID-19 pneumonia treated with hydroxychloroquine and ceftriaxone. She was sedated, paralyzed, and ventilated with volume-controlled ventilation (VCV) with respiratory rate (RR) 35 bpm, tidal volume (TV) 6 mL/kg IBW, FiO2 60%, and PEEP 15 cm H2O with plateau pressure (Ppl) of 27 cm H2O. Inhaled epoprostenol was administered at 25 ng/kg/min. On stable ventilator settings and epoprostenol dose, arterial blood gas (ABG) suggested significant dead-space ventilation with pH 7.12, PaCO2 71 mm Hg, and PaO2 45 mm Hg. d-Dimer was elevated at 5.7 µg/mL (normal < 0.5 µg/mL). Course was complicated by vasodilatory shock requiring norepinephrine 30 mcg/min and vasopressin 2.4 units/h, as well as acute kidney injury (AKI). Given her deterioration, she was treated with tPA 50 mg infusion over 2 h. ABG at the conclusion of the infusion demonstrated marked improvement in alveolar ventilation and oxygenation with pH 7.27, PaCO2 40 mmHg, PaO2 78 mmHg. She was continued on a tPA infusion at 2 mg/h for 24 h with a concomitant heparin drip to achieve therapeutic anticoagulation. Over 24 h, her vasopressor requirement decreased to norepinephrine 4 mcg/min. The patient ultimately died of refractory septic shock 1 week later.
3 CASE 2
A 62-year-old woman with obesity and diabetes who required intubation in the emergency department. VCV was set at RR 30 bpm, TV 6 mL/kg IBW, FiO2 70%, and PEEP 15 cm H2O with Ppl of 25 cm H2O. ABG suggested presence of dead-space ventilation with pH 7.33, PaCO2 55 mm Hg, and PaO2 115 mm Hg. d-Dimer was elevated at 6.1 µg/mL. She had persistent shock requiring norepinephrine 15 mcg/min despite treatment with therapeutic enoxaparin and AKI. She was treated with tPA 50 mg infusion over 2 h. At the conclusion of the infusion, ABG was not changed; however, she had been weaned off of vasopressors. The patient was successfully extubated 10 days later and ultimately discharged home.
4 CASE 3
A 56-year-old man with obesity, hypertension, and diabetes who required initiation of mechanical ventilation in the emergency department. He was ventilated with VCV with RR 30 bpm, TV 6 mL/kg, FiO2 60%, and PEEP 5 cm H2O with Ppl of 14 cm H2O. ABG suggested significant dead-space ventilation with pH 7.14, PaCO2 107 mm Hg and PaO2 84 mm Hg. d-Dimer was elevated to 4.6 µg/mL. The clinical course was notable for AKI and refractory severe shock, requiring norepinephrine 50 mcg/min and vasopressin 2.4 units/h. Echocardiogram demonstrated a hyperdynamic left ventricle, normal right ventricular function, and no clot in transit. Heparin drip to achieve therapeutic anticoagulation was initiated 12 h prior. The heparin drip was discontinued, and he was given tPA 50 mg infusion over 75 min. ABG at the conclusion of the infusion demonstrated improvement in alveolar ventilation, with pH 7.18, PaCO2 89 mm Hg, and PaO2 66 mm Hg, and norepinephrine dose was weaned to 7 mcg/min. Over the ensuing 1 h, the patient became progressively hypoxemic and hypotensive, ultimately suffered a cardiac arrest. Echocardiogram 11 min into resuscitation efforts demonstrated large biventricular thrombi. The patient subsequently expired.
5 CASE 4
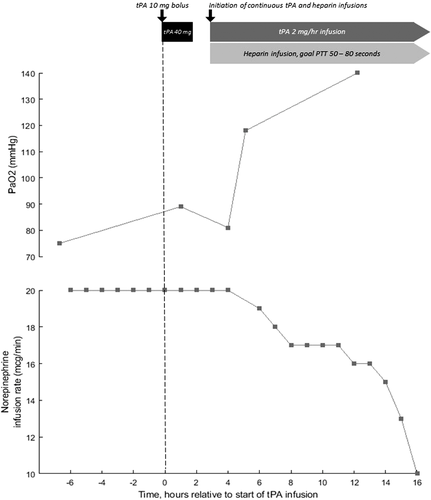
A 57-year-old man with obesity and hypertension. He received convalescent plasma as part of a clinical trial. Course was complicated by vasodilatory shock requiring norepinephrine 10 mcg/min and AKI. He was ventilated with VCV RR 35 bpm, TV 6 mL/kg, FiO2 100%, and PEEP 16 cm H2O with Ppl of 30 cm H2O. ABG indicated significant dead-space ventilation with pH 7.21, PaCO2 51 mm Hg, PO2 81 mm Hg. d-Dimer had increased to 6.6 µg/mL and gas exchange had not improved despite receiving a heparin drip for the preceding 24 h. Given his deterioration, he was administered tPA 50 mg over 2 h without improvement in gas exchange or hemodynamics. He was restarted on a heparin drip to achieve therapeutic anticoagulation and a tPA drip was initiated at 2 mg/h. After 12 h, ABG demonstrated marked improvement in oxygenation with pH 7.27, PaCO2 51 mm Hg, and PaO2 140 mm Hg (Figure 1). The patient ultimately died 9 days from refractory shock.
6 DISCUSSION
We suspect that in many cases the primary mechanism by which COVID-19 causes respiratory failure is pulmonary endothelial dysfunction with diffuse, heterogeneously distributed pulmonary microthrombi in some lung regions, and significant pulmonary vascular dilatation in other lung regions. These simultaneous abnormalities could explain the combined dead-space and shunt physiology, as well as the preserved pulmonary hemodynamics and normal right ventricular function reported previously.3 The coexistence of obliterative lesions and vasodilatory regions is reminiscent of the pathophysiology seen in cirrhotic patients with portopulmonary hypertension (obliterative) and hepatopulmonary syndrome (vasodilatory).4 The improvement in oxygenation noted with increased PEEP in COVID-19 “ARDS” may be attributable to decreased cardiac output, leading to decreased shunt fraction rather than to alveolar recruitment.1
Autopsy studies from the SARS outbreak of the early 2000s, caused by SARS-CoV-1 virus, have demonstrated pulmonary thrombi, pulmonary infarcts, and microthrombi in other organs.5-8 It appears that SARS-CoV-2 is causing similar pathophysiological derangements. Although microthrombi are present in sepsis and classic forms of ARDS, they are unlikely to be the principal cause of respiratory failure and organ dysfunction.9, 10 In COVID-19 pneumonia, the thrombi may play a direct and significant role in gas exchange abnormalities and in multisystem organ dysfunction. The preserved lung compliance noted early in the course of COVID-19 patients with bilateral airspace opacities suggests that the observed pulmonary infiltrates could represent areas of pulmonary infarct and hemorrhage. In our series, disseminated intravascular coagulation was not the cause of microthrombi as all four patients had normal platelet, PT, PTT, and fibrinogen levels without demonstration of hemolysis, despite elevated d-dimer levels. The high prevalence of obesity, hypertension, and diabetes in patients with severe COVID-19 pneumonia may point to an underlying susceptibility to endothelial injury and dysregulation in this metabolic syndrome.
Thrombolysis in this case series had an immediate, physiological impact that variably improved alveolar ventilation, oxygenation, and shock. Thrombolysis improves alveolar ventilation by restoring blood flow to previously occluded regions. This redistribution would reduce blood flow to vasodilated vessels, decreasing the shunt fraction and improving oxygenation. The improvement in shock may be multifactorial, but could be secondary to reperfusion of other ischemic organs that have microthrombi (eg, the kidneys, as all four cases had AKI), leading to an improvement in the overall inflammatory and vasodilatory state. In these four cases, there were no bleeding complications associated with thrombolysis.
These four cases had respiratory failure early in their COVID-19 course and had evidence of the “pulmonary vascular” phenotype (normal compliance, increased dead-space, elevated d-dimers). It may be prudent to consider full systemic anticoagulation for earlier disease to possibly prevent or mitigate progression of the syndrome. As this phenotype progresses, and patients develop severe progressive cardiopulmonary compromise, therapeutic anticoagulation alone may not be effective and thus systemic thrombolysis may be beneficial; Cases 3 and 4 had been receiving therapeutic anticoagulation prior to tPA administration without noticeable improvement. A second more classic “ARDS” phenotype may exist as a discrete entity or as part of a spectrum of disease with low compliance from continued lung injury due to mechanical ventilation or COVID-19 induced lung injury.
7 CONCLUSIONS
The pathophysiology of COVID-19 severe respiratory failure may be driven by pulmonary vascular endothelial dysfunction and thrombosis that responds to thrombolysis and anticoagulation. These therapeutic approaches should be considered in the management of COVID-19 patients and must be further examined in clinical research studies.
AUTHOR CONTRIBUTIONS
H.P. was associated with concept, clinical care, data gathering, interpretation, and drafting of the manuscript; C.E.V. was associated with concept, interpretation, and revision of the manuscript; T.T. was associated with clinical care, data gathering, interpretation, and revision of the manuscript; G.C. was associated with clinical care, data gathering, interpretation, and revision of the manuscript; G.S. was associated with interpretation and revision of the manuscript; A.Z. was associated with interpretation and revision of the manuscript; N.D. was associated with clinical care; J.O. was associated with interpretation and revision of the manuscript; R.K.S. was associated with clinical care, interpretation, and revision of the manuscript; C.A.P. was associated with concept, clinical care, interpretation, and revision of the manuscript
ACKNOWLEDGMENT
C.E.V. was supported by grant R01 HL141268.




