Altered lipidomic profiles in patients with and without osteonecrosis of the femoral head after 1-month glucocorticoid treatment
Glucocorticoids (GCs) are widely applied in clinical work, but high-dose or long-term GC therapies are associated with osteonecrosis of the femoral head (ONFH). Although early-stage glucocorticoid-associated osteonecrosis of the femoral head (GA-ONFH) can be asymptomatic, it usually progresses to disability status unless early diagnosis and treatment. Dysfunction of lipid metabolism is long believed playing a crucial role in GA-ONFH.1 Clinical lipidomics is a novel high-throughput approach to discover disease-specific biomarkers and molecular mechanisms. However, the lipidomic profiles of GA-ONFH remain unknown because of a lack of early-stage patients and proper controls to avoid bias from influence of GC on lipid metabolism. Thus, the present study investigated serum lipidomic profiles of patients with and without GA-ONFH at the time both before and after initial GC treatment. To our knowledge, it is the first clinical study on circulating lipidomic profiles of GA-ONFH, and the first to reveal altered lipidomic profiles due to initial short-term GC treatment.
Based on a previously reported cohort,2 the present study was designed as a prospective nested case--control study. Patients with autoimmune diseases who were anticipated to start initial systemic GC therapy were enrolled. Seven patients diagnosed with GA-ONFH after short-term GC treatment (67 ± 18 days), and 11 patients who accepted similar treatment but were confirmed without osteonecrosis after long-term follow-ups (35 ± 1 months) were included (Table 1). Their serum specimens before and after 1-month treatment were collected for lipidomics measurement.
| Patient | Age | Gender | Basic disease | Time before necrosisa | GD1Mb |
|---|---|---|---|---|---|
| Necrosis | 36.6 ± 1.8 | 67 ± 18 days | 1763 ± 243 | ||
| N-1 | 33 | Female | AOSD | 130 days | 1350 |
| N-2 | 38 | Female | AOSD | 42 days | 1800 |
| N-3 | 46 | Female | SLE | 34 days | 2790 |
| N-4 | 33 | Female | SLE | 64 days | 1800 |
| N-5 | 38 | Female | SLE | 34 days | 2350 |
| N-6 | 34 | Female | SLE | 30 days | 1350 |
| N-7 | 34 | Male | NS | 137 days | 900 |
| Control | 37.5 ± 5.0 | 35 ± 1 months | 1776 ± 139b | ||
| C-1 | 45 | Female | SLE | 43 months | 1200 |
| C-2 | 22 | Female | AOSD | 38 months | 2065 |
| C-3 | 47 | Female | SLE | 30 months | 2198 |
| C-4 | 23 | Female | SLE | 34 months | 1775 |
| C-5 | 17 | Female | SLE | 38 months | 2250 |
| C-6 | 19 | Female | AOSD | 31 months | 2300 |
| C-7 | 56 | Female | SLE | 37 months | 2160 |
| C-8 | 26 | Female | SLE | 37 months | 1500 |
| C-9 | 64 | Female | SLE | 36 months | 1085 |
| C-10 | 45 | Male | NS | 32 months | 1800 |
| C-11 | 49 | Male | NS | 31 months | 1200 |
- Abbreviations: AOSD, adult onset still disease; NS, nephrotic syndrome; SLE, systemic lupus erythematosus.
- a Time before diagnosed with osteonecrosis in the GA-ONFH group or time of follow-up without osteonecrosis in the control group.
- b GD1M: Glucocorticoid dose in the first month (prednisone-equivalent dose/mg); data are represented as mean ± SEM.
Lipid extraction and measurement was finished as reported previously.3 The significance level for univariate analysis was at p < 0.05. As for multivariate analysis, orthogonal partial least squares-discriminant analysis (OPLS-DA) was conducted to select key metabolites through variable importance in projection (VIP) values. Lipid elements with p-values less than 0.05 were considered as significantly differential metabolites if their fold changes were greater than 1.5, less than 1/1.5, or VIP values were greater than 1.0.
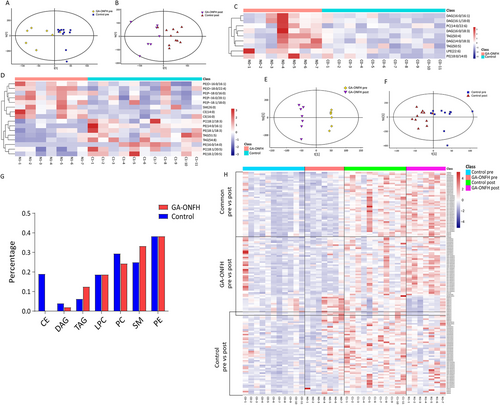
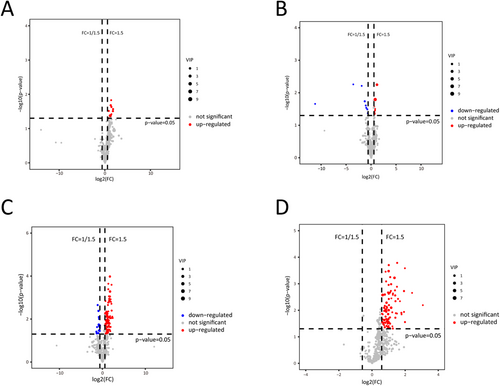
Clear separations between two groups were observed in the OPLS-DA models both before and after GC treatment (Figure 1A and B). Nine differential elements before treatment and 16 after treatment were identified (Table 2, Figures 1C and D and 2A and B). As for the altered lipidomic profiles after treatment, OPLS-DA models showed clear separations in both groups, especially in the GA-ONFH group (Figure 1E and F). Totally, 93 and 95 altered elements were identified in GA-ONFH and control groups, respectively (Figure 2C and D). Among all the altered elements, 42 lipids with same variation tendency in both groups were considered as altered lipids due to 1-month GC treatment (Table 3). The other altered elements specifically appeared in one group might be associated with the process of osteonecrosis, including 51 elements in the GA-ONFH group and 53 elements in the control group (Table 4). As shown in Figure 1G, two groups had similar variation tendency on the 42 altered lipids due to GC treatment, but distinguished from each other when considering the 102 altered lipids potentially associated with GA-ONFH. Figure 1H showed the percentage of altered elements in each class of lipids.
| Before GC treatment | After GC treatment | ||||||
|---|---|---|---|---|---|---|---|
| Elements | Foldsa | VIP | p value | Elements | Foldsa | VIP | p value |
| DAG(16:1/18:0) | 3.40 | 0.26 | 0.03 | CE(14:0) | 2.20 | 1.62 | 0.01 |
| DAG(16:0/16:1) | 3.33 | 0.42 | 0.03 | PE(P-16:0/20:1) | 1.76 | 0.06 | 0.02 |
| PE(18:0/14:0) | 2.67 | 0.02 | 0.04 | PE(O-16:0/16:1) | 1.75 | 0.02 | 0.03 |
| DAG(16:0/18:3) | 2.64 | 0.22 | 0.02 | PE(P-18:1/18:0) | 1.71 | 0.04 | 0.04 |
| DAG(14:0/18:3) | 2.56 | 0.05 | 0.01 | CE(16:0) | 1.70 | 4.34 | 0.02 |
| TAG(50:4) | 2.37 | 0.58 | 0.02 | PE(O-18:0/22:4) | 1.65 | 0.05 | 0.04 |
| LPE(22:6) | 2.18 | 2.80 | 0.04 | SM(26:0) | 1.58 | 0.05 | 0.04 |
| PC(14:0/22:6) | 1.77 | 0.20 | 0.03 | PE(P-18:0/16:0) | 1.53 | 0.03 | 0.03 |
| TAG(50:5) | 1.71 | 0.20 | 0.04 | PE(16:0/14:0) | 4.21 × 10–4 | 4.0 × 10–3 | 0.02 |
| PE(14:0/16:1) | 8.40 × 10–2 | 9.8 × 10–3 | 0.01 | ||||
| PE(18:2/20:5) | 0.268 | 0.02 | 0.01 | ||||
| TAG(54:8) | 0.408 | 0.36 | 0.02 | ||||
| TAG(51:5) | 0.473 | 0.14 | 0.02 | ||||
| PE(18:1/18:3) | 0.503 | 0.08 | 0.03 | ||||
| PC(18:2/18:3) | 0.586 | 0.21 | 0.03 | ||||
| PC(18:1/20:5) | 0.629 | 0.18 | 0.04 | ||||
- Abbreviations: CE, cholesteryl ester; DAG, diacylglyceride; LPE, lysophosphatidylethanolamine; PC, phosphatidylcholine; PE, phosphatidylethanolamine; SM, sphingomyelin; TAG, triacylglycerol; VIP, variable influence in projection.
- a Compared GA-ONFH group above the control group.
| GA-ONFH group | Control group | |||||
|---|---|---|---|---|---|---|
| Elements | Foldsa | VIP | p value | Foldsa | VIP | p value |
| LPC(18:2) | 1.76 | 2.02 | 0.01 | 1.54 | 1.99 | 0.00 |
| LPC(22:5) | 1.85 | 0.10 | 0.02 | 1.59 | 0.11 | 0.03 |
| PC(14:0/18:2) | 1.97 | 1.01 | 0.00 | 2.21 | 1.40 | 0.01 |
| PC(14:0/18:3) | 1.71 | 0.13 | 0.04 | 2.65 | 0.25 | 0.00 |
| PC(14:0/20:2) | 2.25 | 0.18 | 0.01 | 2.37 | 0.19 | 0.04 |
| PC(14:0/20:4) | 2.02 | 0.40 | 0.01 | 2.21 | 0.54 | 0.00 |
| PC(14:0/22:4) | 2.60 | 0.13 | 0.03 | 2.03 | 0.14 | 0.04 |
| PC(16:0/20:1) | 1.80 | 0.34 | 0.01 | 1.52 | 0.29 | 0.03 |
| PC(16:0/20:5) | 1.93 | 0.87 | 0.04 | 1.99 | 1.24 | 0.00 |
| PC(18:2/18:2) | 1.93 | 1.71 | 0.02 | 1.76 | 2.12 | 0.01 |
| PC(18:2/20:4) | 1.87 | 0.76 | 0.03 | 1.82 | 1.06 | 0.01 |
| PC(18:2/20:5) | 2.54 | 0.19 | 0.00 | 2.60 | 0.22 | 0.00 |
| PE(14:0/22:6) | 2.17 | 0.03 | 0.02 | 3.94 | 0.05 | 0.00 |
| PE(18:0/18:0) | 1.61 | 0.15 | 0.03 | 1.79 | 0.23 | 0.00 |
| PE(18:2/16:1) | 4.35 | 0.24 | 0.00 | 2.85 | 0.23 | 0.00 |
| PE(O-16:0/18:1) | 2.47 | 0.12 | 0.00 | 1.65 | 0.10 | 0.04 |
| PE(O-16:0/20:5) | 1.66 | 0.05 | 0.03 | 1.91 | 0.06 | 0.03 |
| PE(O-18:0/20:5) | 2.32 | 0.06 | 0.01 | 1.95 | 0.08 | 0.03 |
| PE(P-16:0/16:0) | 2.58 | 0.08 | 0.01 | 1.82 | 0.06 | 0.03 |
| PE(P-16:0/16:1) | 2.33 | 0.07 | 0.01 | 1.96 | 0.06 | 0.03 |
| PE(P-16:0/18:1) | 2.95 | 0.46 | 0.00 | 1.73 | 0.33 | 0.03 |
| PE(P-16:0/18:2) | 2.97 | 0.92 | 0.00 | 1.92 | 0.71 | 0.03 |
| PE(P-16:0/20:5) | 3.49 | 0.15 | 0.04 | 3.95 | 0.21 | 0.03 |
| PE(P-16:0/22:5) | 2.43 | 0.47 | 0.02 | 2.00 | 0.42 | 0.00 |
| PE(P-16:0/22:6) | 1.68 | 0.33 | 0.04 | 1.81 | 0.43 | 0.01 |
| PE(P-16:1/18:1) | 2.20 | 0.03 | 0.01 | 3.42 | 0.05 | 0.01 |
| PE(P-18:0/16:1) | 2.82 | 0.09 | 0.00 | 2.32 | 0.08 | 0.01 |
| PE(P-18:0/18:0) | 3.11 | 0.06 | 0.02 | 1.77 | 0.05 | 0.01 |
| PE(P-18:0/18:1) | 3.57 | 0.50 | 0.00 | 2.02 | 0.37 | 0.01 |
| PE(P-18:0/18:2) | 3.32 | 1.13 | 0.00 | 1.95 | 0.83 | 0.01 |
| PE(P-18:0/22:5) | 2.32 | 0.35 | 0.01 | 2.11 | 0.31 | 0.00 |
| PE(P-18:0/22:6) | 2.08 | 0.41 | 0.04 | 1.94 | 0.45 | 0.00 |
| PE(P-18:1/16:1) | 2.56 | 0.07 | 0.00 | 2.06 | 0.06 | 0.02 |
| PE(P-18:1/18:1) | 3.31 | 0.41 | 0.00 | 1.88 | 0.30 | 0.02 |
| PE(P-18:1/18:2) | 3.27 | 0.80 | 0.00 | 1.87 | 0.60 | 0.03 |
| PE(P-18:1/20:4) | 2.25 | 0.82 | 0.02 | 2.00 | 0.90 | 0.01 |
| PE(P-18:1/22:5) | 1.98 | 0.20 | 0.02 | 1.99 | 0.23 | 0.00 |
| PE(P-18:2/18:2) | 3.19 | 0.22 | 0.00 | 1.98 | 0.20 | 0.03 |
| PE(P-18:2/20:4) | 2.00 | 0.19 | 0.01 | 2.01 | 0.26 | 0.00 |
| SM(14:0) | 1.90 | 0.81 | 0.00 | 1.69 | 0.76 | 0.00 |
| SM(22:0) | 1.65 | 1.50 | 0.03 | 1.43 | 1.39 | 0.00 |
| SM(22:1) | 1.56 | 1.19 | 0.01 | 1.35 | 1.15 | 0.03 |
- Abbreviation: VIP, variable influence in projection.
- a Compared lipid levels after 1-month GC treatment above those before GC treatment.
| GA-ONFH group | Control group | ||||||
|---|---|---|---|---|---|---|---|
| Elements | Foldsa | VIP | p value | Elements | Foldsa | VIP | p value |
| LPC(20:5) | 2.39 | 0.11 | 0.00 | LPC(14:0) | 1.68 | 0.35 | 0.02 |
| PC(14:0/18:1) | 1.93 | 1.00 | 0.03 | PC(14:0/14:0) | 2.50 | 0.17 | 0.04 |
| PC(14:0/20:3) | 2.64 | 0.38 | 0.02 | PC(14:0/20:1) | 2.78 | 0.07 | 0.002 |
| PC(14:0/22:6) | 2.13 | 0.29 | 0.00 | PC(16:0/14:0) | 2.03 | 0.55 | 0.01 |
| PC(16:0/18:3) | 1.66 | 1.13 | 0.02 | PC(16:0/18:2) | 1.36 | 4.02 | 0.03 |
| PC(16:0/20:4) | 1.38 | 3.62 | 0.02 | PC(16:0/22:4) | 1.51 | 0.59 | 0.02 |
| PC(18:0/20:5) | 1.65 | 0.61 | 0.01 | PC(18:0/18:3) | 0.510 | 0.54 | 0.04 |
| PC(18:1/18:2) | 1.41 | 1.99 | 0.04 | PC(18:2/20:2) | 1.60 | 0.23 | 0.03 |
| PC(18:1/18:3) | 1.83 | 0.41 | 0.01 | PC(18:2/22:4) | 1.56 | 0.08 | 0.04 |
| PC(18:1/20:5) | 2.00 | 0.35 | 0.00 | PC(20:0/20:2) | 4.08 × 104 | 0.05 | 0.04 |
| PC(18:2/18:3) | 2.62 | 0.40 | 0.00 | PE(14:0/20:3) | 2.53 | 0.02 | 0.04 |
| PC(18:2/20:3) | 2.11 | 0.63 | 0.03 | PE(16:0/20:1) | 1.60 | 0.04 | 0.00 |
| PC(20:0/20:4) | 2.11 | 0.28 | 0.02 | PE(18:1/16:1) | 2.05 | 0.07 | 0.04 |
| PC(20:0/20:5) | 3.79 | 0.08 | 0.01 | PE(O-16:0/18:2) | 2.07 | 0.16 | 0.04 |
| PE(14:0/16:1) | 8.35 | 0.01 | 0.01 | PE(O-16:0/20:1) | 3.18 | 0.04 | 0.03 |
| PE(14:0/20:4) | 1.82 | 0.05 | 0.04 | PE(O-16:0/20:2) | 3.67 | 0.13 | 0.02 |
| PE(14:0/22:5) | 3.20 | 0.03 | 0.00 | PE(O-16:0/22:4) | 2.76 | 0.17 | 0.01 |
| PE(16:0/20:5) | 1.58 | 0.08 | 0.03 | PE(O-16:0/22:5) | 3.07 | 0.82 | 0.02 |
| PE(18:0/20:5) | 1.56 | 0.13 | 0.01 | PE(O-18:0/16:0) | 2.10 | 0.05 | 0.04 |
| PE(18:1/18:3) | 1.66 | 0.08 | 0.03 | PE(O-18:0/20:1) | 2.67 | 0.02 | 0.02 |
| PE(18:1/20:5) | 1.85 | 0.06 | 0.02 | PE(O-18:0/22:4) | 2.33 | 0.11 | 0.01 |
| PE(18:2/18:3) | 2.83 | 0.06 | 0.01 | PE(P-16:0/18:0) | 2.57 | 0.06 | 0.00 |
| PE(18:2/20:5) | 5.32 | 0.03 | 0.00 | PE(P-16:0/20:1) | 3.85 | 0.10 | 0.00 |
| PE(18:2/22:5) | 2.38 | 0.03 | 0.00 | PE(P-16:0/20:2) | 3.91 | 0.11 | 0.01 |
| PE(O-16:0/18:0) | 2.24 | 0.02 | 0.00 | PE(P-16:0/20:3) | 2.74 | 0.39 | 0.02 |
| PE(O-16:0/18:3) | 2.10 | 0.04 | 0.04 | PE(P-16:0/22:4) | 2.27 | 0.36 | 0.01 |
| PE(O-16:0/22:6) | 1.86 | 0.19 | 0.01 | PE(P-18:0/16:0) | 2.39 | 0.12 | 0.00 |
| PE(O-18:0/18:0) | 2.00 | 0.02 | 0.04 | PE(P-18:0/20:1) | 4.85 | 0.08 | 0.01 |
| PE(O-18:0/18:3) | 1.93 | 0.04 | 0.04 | PE(P-18:0/20:2) | 3.35 | 0.11 | 0.01 |
| PE(O-18:0/20:4) | 1.70 | 0.18 | 0.01 | PE(P-18:0/20:3) | 2.68 | 0.48 | 0.01 |
| PE(O-18:0/22:5) | 1.76 | 0.09 | 0.00 | PE(P-18:0/22:4) | 2.28 | 0.24 | 0.04 |
| PE(O-18:0/22:6) | 1.51 | 0.09 | 0.01 | PE(P-18:1/16:0) | 3.38 | 0.21 | 0.01 |
| PE(P-16:0/18:3) | 2.70 | 0.10 | 0.01 | PE(P-18:1/18:0) | 4.20 | 0.07 | 0.00 |
| PE(P-16:0/20:4) | 1.97 | 0.86 | 0.02 | PE(P-18:1/20:1) | 5.16 | 0.07 | 0.01 |
| PE(P-18:0/18:3) | 2.82 | 0.12 | 0.00 | PE(P-18:1/20:2) | 4.31 | 0.11 | 0.00 |
| PE(P-18:0/20:4) | 1.98 | 1.10 | 0.01 | PE(P-18:1/20:3) | 2.98 | 0.35 | 0.01 |
| PE(P-18:0/20:5) | 3.78 | 0.33 | 0.00 | PE(P-18:1/22:4) | 1.89 | 0.14 | 0.02 |
| PE(P-18:1/18:3) | 2.95 | 0.09 | 0.00 | DAG(18:1/20:4) | 0.388 | 0.31 | 0.04 |
| PE(P-18:1/20:5) | 3.40 | 0.21 | 0.01 | SM(24:0) | 1.63 | 1.28 | 0.02 |
| PE(P-18:1/22:6) | 1.75 | 0.34 | 0.02 | TAG(50:4) | 0.372 | 0.55 | 0.04 |
| PE(P-18:2/22:6) | 2.19 | 0.12 | 0.00 | TAG(50:5) | 0.569 | 0.26 | 0.01 |
| CE(16:1) | 1.96 | 2.80 | 0.03 | TAG(51:4) | 0.566 | 0.34 | 0.02 |
| CE(18:1) | 1.26 | 4.30 | 0.02 | TAG(51:5) | 0.414 | 0.15 | 0.02 |
| CE(18:3) | 1.46 | 2.10 | 0.04 | TAG(52:4) | 0.595 | 3.04 | 0.02 |
| CE(20:5) | 2.53 | 1.45 | 0.00 | TAG(52:5) | 0.542 | 1.36 | 0.02 |
| DAG(14:0/22:6) | 2.34 | 0.03 | 0.04 | TAG(52:6) | 0.555 | 0.41 | 0.01 |
| DAG(18:2/20:5) | 1.80 | 0.11 | 0.02 | TAG(53:4) | 0.641 | 0.25 | 0.04 |
| TAG(48:4) | 1.69 | 0.48 | 0.04 | TAG(54:3) | 0.569 | 1.43 | 0.03 |
| TAG(48:5) | 1.86 | 0.16 | 0.04 | TAG(54:7) | 0.519 | 0.69 | 0.00 |
| TAG(50:5) | 1.53 | 0.32 | 0.02 | TAG(54:8) | 0.457 | 0.34 | 0.00 |
| TAG(50:6) | 2.00 | 0.08 | 0.04 | TAG(56:5) | 0.656 | 0.30 | 0.02 |
| TAG(51:5) | 1.65 | 0.15 | 0.01 | ||||
| TAG(53:0) | 1.51 | 0.27 | 0.03 | ||||
- Abbreviation: VIP, variable influence in projection.
- a Compared lipid levels after 1-month GC treatment above those before GC treatment.
Higher concentrations of triacylglycerol (TAG) and diacylglyceride (DAG) were observed in GA-ONFH group before treatment. Consistent with previous study,4 it indicated that higher serum concentration and metabolic level of TAG might be risk factors for GA-ONFH. Through current research, GC can modulate both TAG synthesis and hydrolysis. The effect of GC on circulating TAG may differ due to dose of GC and length of treatment.4, 5 Intriguingly, the present study showed that TAGs and DAGs significantly increased in the control group but decreased in the GA-ONFH group after 1-month GC treatment. A previous study,6 simultaneously focusing on plasma TAG and hepatic steatosis in a rat model of GA-ONFH, provided with a potential explanation. It was found that plasma TAG decreased in the first 3 weeks after GC injection but increased in the fourth week. Along with the lowest plasma TAG, the most severe hepatic steatosis was observed in the second and third weeks. Considering the pathological characterization of fat accumulation in the medullary cavity of GA-ONFH, we hypothesized that circulating TAG might decrease to a greater degree or for longer period in GA-ONFH patients because of a more severe lipid accumulation in the cancellous bone including femoral heads.
Though several cholesteryl esters (CEs) increased significantly in the control group due to GC treatment, CE(14:0) and CE(16:0), with fairly high VIP values and fold changes, were much higher in the GA-ONFH group after GC treatment. It consisted with previous studies that GC treatment could elevate serum cholesterol rapidly in GA-ONFH patients when comparing with those without ONFH, especially in the first month.4, 7 Glycerophospholipids, phosphatidylethanolamines (PEs) in particular, occupied a large part of the differential lipids between the two groups. Likewise, previous studies reported that glycerophospholipids were distinguished in the bone trabecula8 and plasma9 of ONFH patients when comparing with healthy controls.
Though with accumulating studies on the effect of GCs on lipidomic profiles, there is a lack of studies on the lipidomic profiles before and after GC treatment within a same patient population. An enlightening study10 compared the lipidomic profiles after 8-month GC treatment with those before or within 2-week GC treatment in eight patients with dermatomyositis and polymyositis. As the first study to reveal the altered lipidomic profiles due to initial short-term GC treatment, the present study showed a comprehensive increase of phospholipid, generally similar to the former study.10 Considering the great impact of GCs on lipid metabolism, one suggestion is that proper control groups with patients once accepted GC treatment is necessary for studies on GA-ONFH.
By employing lipidomics analysis, the present study revealed serum lipidomic profiles of GA-ONFH and altered lipidomic profiles due to 1-month GC treatment for the first time. Higher LPE(22:6) before treatment and higher CE(14:0), CE(16:0) after 1-month GC treatment were considered highly associated with early-stage GA-ONFH. On the other hand, higher concentration of TAG and DAG before treatment and a decrease of TAG after 1-month treatment were considered as risk factors for GA-ONFH. However, as a pilot study, these biomarkers and risk factors need to be validated in larger scale studies. The disease-specific lipidomic profiles may also offer new ideas for future studies.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the support provided by the departments of Rheumatology and Nephrology, Zhongshan Hospital, Shanghai, China. Special thanks to the participant patients for their cooperation.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
FUNDING INFORMATION
National Natural Science Foundation of China; Grant Numbers: 81672157 and 81871742; Shanghai Hospital Development Center Emerging Advanced Technology Joint Research Project; Grant Number: SHDC12017107; Three-year Action Plan Major Clinical Research Project; Grant Number SHDC2020CR3075B.
DATA AVAILABILITY STATEMENT
Data are available on reasonable request from the corresponding author.
ETHICS APPROVAL
This study complies with the Declaration of Helsinki and was approved by the Ethics Committee of Zhongshan Hospital, Shanghai, China [B2013-124(2)]. All patients provided a written informed consent.




