Inhibition of proteasomal deubiquitinases USP14 and UCHL5 overcomes tyrosine kinase inhibitor resistance in chronic myeloid leukaemia
Liling Jiang, Qingyan He and Xin Chen contributed equally to this work.
Abstract
Background
Chronic myeloid leukaemia (CML) is a haematological cancer featured by the presence of BCR-ABL fusion protein with abnormal tyrosine kinase activation. Classical tyrosine kinase inhibitor (TKI)-based therapies are available to patients with CML. However, acquired resistance to TKI has been a challenging obstacle, especially stubborn T315I mutation is the most common cause. Therefore, it is especially urgent to find more effective targets to overcome TKI resistance induced by BCR-ABLT315I. Proteasomal deubiquitinases (USP14 and UCHL5) have fundamental roles in the ubiquitin-proteasome system and possess multiple functions during cancer progression.
Methods
The human peripheral blood mononuclear cells were collected to measure the mRNA expression of USP14 and UCHL5, as well as to detect the toxicity effect of b-AP15. We explored the effect of b-AP15 on the activity of proteasomal deubiquitinases. We detected the effects of b-AP15 on BCR-ABLWT and BCR-ABLT315I CML cells in vitro and in the subcutaneous tumour model. We knocked down USP14 and/or UCHL5 by shRNA to explore whether these proteasomal deubiquitinases are required for cell proliferation of CML.
Results
In this study, we found that increased expression of the proteasomal deubiquitinase USP14 and UCHL5 in primary cancer cells from CML patients compared to healthy donors. b-AP15, an inhibitor of USP14 and UCHL5, exhibited potent tumour-killing activity in BCR-ABLWT and BCR-ABLT315I CML cell lines, as well as in CML xenografts and primary CML cells. Mechanically, pharmacological or genetic inhibition of USP14 and UCHL5 induced cell apoptosis and decreased the protein level of BCR-ABL in CML cells expressing BCR-ABLWT and BCR-ABLT315I. Moreover, b-AP15 synergistically enhanced the cytotoxic effect caused by TKI imatinib in BCR-ABLWT and BCR-ABLT315I CML cells.
Conclusion
Collectively, our results demonstrate targeting USP14 and UCHL5 as a potential strategy for combating TKI resistance in CML.
1 BACKGROUND
Chronic myeloid leukaemia (CML) is a haematopoietic malignancy, mainly caused by the formation of fusion gene BCR-ABL, which is generated from t(9;22) chromosome translocation.1 Therefore, selective BCR-ABL tyrosine kinase inhibitor (TKI) is a classic strategy for CML therapy. Nevertheless, the T315I gatekeeper mutant impairs the binding between TKI and the ATP-pocket of BAR-ABL, conferring cell resistant to first- and second-generation TKI (e.g. imatinib, nilotinib and dasatinib) in clinics.2 In order to override this type of mutation, a third-generation TKI, ponatinib, has been exploited. However, ponatinib's severe cardiovascular toxicity profile limits its clinical application.3 Thus, additional strategies to overcome T315I resistance are desperately needed.
Since tumour cells are characterised by abnormal metabolism, the ubiquitin-proteasome system (UPS) that mainly functions in intracellular protein turnover is highly active. A paramount example of this problem is the observation that specific inhibitors targeting the UPS represents an effective strategy for cancer therapy.4, 5 The integrated 26S proteasome is comprised of two 19S regulation portions and a core 20S degradation portion. Before an ubiquitinated protein is degraded by the proteasome, it should be firstly recruited to 19S portion, where its polyubiquitin chain is removed by 19S proteasomal deubiquitinases, including USP14, UCHL5 and POH1.6 The first 20S proteasome inhibitor, bortezomib, has been approved by FDA for the treatment of multiple myeloma.7, 8 However, acquired resistance of bortezomib hampers its therapeutic efficacy. Fortunately, pharmacological inhibition of proteasomal deubiquitinases provides an alternative strategy to interfering with protein degradation by the proteasome.6 Therefore, proteasomal deubiquitinases are now considered as promising candidates for antitumour strategy.9
b-AP15 is an inhibitor of proteasomal deubiquitinases, including USP14 and UCHL5.10 The antineoplastic effects of b-AP15 have been shown to be related to activation of acute proteotoxic stress response and production of reactive oxygen species.11-14 Accordingly, b-AP15 shows a significant antineoplastic effect in both solid and nonsolid tumours, including oesophageal squamous cell carcinoma, neuroblastoma and multiple myeloma.15-18 However, the effects of proteasomal deubiquitinase inhibitor on CML have not yet been reported.
In this study, we revealed the potential of targeting proteasomal deubiquitinase as a therapeutic strategy in TKI-resistant CML. We demonstrated that targeting USP14 and UCHL5 by b-AP15 showed obvious antitumour efficiency in BCR-ABLWT and BCR-ABLT315I CML cell lines and xenografts, as well as in primary CML cells. Furthermore, we found that b-AP15 synergised imatinib to induce cell death in TKI-resistant CML cells. These findings have important implications for improving therapies aimed at overcoming TKI resistance in CML.
2 MATERIALS AND METHODS
2.1 Cell lines and PBMCs
KBM5 cells expressed BCR-ABLWT was isolated from a CML patient in blast crisis.19 KBM5-T315I cells were derived from KBM5 by long-term exposing to imatinib and selecting the survival clones harbouring the BCR-ABLT315I.20 BaF3 stably expressing BCR-ABLWT or BCR-ABLT315I were kindly provided by B.Z Carter (University of Texas M.D. Anderson Cancer Center, USA). K562 cells were purchased from ATCC. KBM5 and KBM5-T315I were cultured in IMDM medium with 10% FBS. BaF3, BaF3-T315I, and K562 cells were cultured in RPMI 1640 medium with 10% FBS.
PBMCs were collected from the Hematology Department, the First Affiliated Hospital of Guangzhou Medical University, which was approved by the medical research ethics committee review (approve number: 2022–77), with the permission of the patients. PBMCs were isolated and cultured in RPMI 1640 medium with 15% FBS.
2.2 Chemicals and reagents
The small molecules b-AP15, ZVAD, imatinib and bortezomib were purchased from Selleck Chemicals and dissolved in DMSO and stored at –20°C. The final dose of DMSO limited to 0.3% in all experiments. The antibodies as follows were obtained from Cell Signaling Technology (Danvers, MA): PARP (#9532S), p27 (#3688S), USP14 (#11931S), Caspase-3 (#9662S), Caspase-8 (#9746S), Caspase-9 (#9508S), XIAP (#2042S), Bax (#5023), Bcl-2 (15071S), Survivin (#2808S), AIF (#4642S), Cytochrome C (#4272S), PERK (#5683S), ATF4 (#11815S), CHOP (#2895), BCR-ABL (#2862S), p-BCR-ABL (#2861S), STAT5 (#9363S), p-AKT (#2965), AKT (#4685), CRKL (#3182S) and p-CRKL (#3181S). The antibodies as follows were obtained from Proteintech (Rosemont, USA): Mcl-1 (#16225-1-Ap) and BCL-xl (#66020-I-Ig). The antibodies as follows are purchased from Abcam (Cambridge, MA): Cleaved Caspase 9 (#AB3629) and Cleaved Caspase 3 (#AB2302). The antibody of ubiquitin was purchased from Santa Cruz Biotechnology. p-STAT5 (#50095), anti-mouse (12-349) and anti-rabbit (AP132P) antibodies were obtained from Merk Millipore (Darmstadt, Germany). All the above antibodies were used at a dilution of 1:1000.
2.3 Western blots
Western blots were performed as previously described.20 In brief, cells were collected and washed twice with PBS. Whole-cell lysate is generated using RIPA buffer (Beyotime Biotechnology, Shanghai, China) supplemented with PMSF and Protease Inhibitor Cocktail (Roche, Indianapolis, IN). The bicinchoninic acid (BCA) assay was used to determine the total protein concentration.
2.4 qPCR
- USP14 forward, 5′-CAGCTGTTTGCGTTGACTGG-3′
- USP14 reverse, 5′-GAAGAGCATCTGCTGACCCC-3′
- UCHL5 forward, 5′-GGCTCTGTGGTTCAGGACTC-3′
- UCHL5 reverse, 5′-TGGACATCCTGGTGGGTACA-3′
- POH1 forward, 5′-GAGGCAAGACAAGGGTCCATC-3′
- POH1 reverse, 5′-TTCTGGCAGGTACAACTTCCC-3′
2.5 Proteasomal activity assay
In brief, cells were lysed in assay buffer (25 mM Tris-HCl, pH 7.4), and then incubated with b-AP15 or imatinib on ice for 30 min. To evaluate the proteasome's chymotrypsin-like (CT-like), caspase-like (C-like), and trypsin-like activity (T-like), cell lysis was incubated with Suc-LLVY-AMC, or Z-LLE-AMC or Boc-LRR-AMC for 2 h in the dark at 37°C.
2.6 HA-Ub-VS assay
HA-Ub-VS is a probe targeting the deubiquitinase active site and can be used to test the binding capacity of compounds to deubiquitinase. Cells were harvested after incubated 3 h with b-AP15, lysed in a lysis buffer (25 mM Tris-HCl, 20 mM NaCl, 5 mM MgCl2, 200 µM ATP, pH7.4) for 30 min. The equal amounts of protein from each sample were incubated with HA-Ub-VS (1 µM) in 37°C for 30 min. Finally, the HA-Ub-VS labelled proteins were measured by Western blots.
2.7 Cell viability
A total of 2 × 104 cells/well were seeded in 96 well plates with a volume of 100 µl and were stimulated with b-AP15 for 48 h and incubated with 20 µl MTS (Promega, Madison, WI) at 37°C for 2 h. Then, the absorbance was measured by a microplate reader at wavelength 490 nm.
2.8 Cell apoptosis
Cell apoptosis was investigated using Annexin V/PI double staining kit (Sungene Biotech, Tianjin, China). The cells were harvested after the treatment of b-AP15 for 24 h, and washed twice with ice-cold PBS, followed by incubation on ice for 5 min with 5 µl Annexin V. Then, the cells were incubated with PI for 10 min, followed with a flow cytometer and Cytexpert software analysis.
2.9 Mitochondrial membrane permeability
The mitochondrial membrane potential was determined by rhodamine 123 (Merck, Darmstadt, Germany) staining. Briefly, cells were stimulated with b-AP15 for 24 h, and collected for rhodamine 123 staining. Then, cells were resuspended in 500 µl cell culture medium, and incubated with 2.5 µg/ml rhodamine 123 for 30 min at 37°C in the dark. Finally, the mitochondrial membrane potential of cells was measured by flow cytometer and analysed by Cytexpert software.
2.10 Xenograft model
Five-week-old nude Balb/c mice were raised and housed in the animal facility of Guangzhou Medical University. Inoculated subcutaneously on the mice with 1 × 107 cells of CML cells, every cell type includes 12 mice. The mice were randomly separated into a vehicle and b-AP15 after inoculated, and then intraperitoneal injection with vehicle (20% Cremophor EL, 20% PEG400, and 40% Saline) or 5 mg/kg/day b-AP15 every day. The mice body weight and tumour size were recorded, and the tumour volume was calculated as previously described.20 The mice were sacrificed when the maximum tumour reached 1500 mm3, then the tumour tissue that was isolated from the mice body and weighted. The tumour tissue was lysed for western blot analysis or fixed for immunohistochemical staining. The experiments were subjected to the approval of the Institutional Animal Care and Use Committee of Guangzhou Medical University and following the ethical rules of animal experiments (GY2019-109).
2.11 Immunohistochemical staining
4% paraformaldehyde-fixed xenografts were used to prepare the paraffin section according to a standard method. Paraffin sections were immunostained for K48-linkage ubiquitin, Ki67 and BCR-ABL. The freshly prepared DAB solution (Beyotime, Shanghai, China) was used to detect the reaction products. Then, haematoxylin was used to counterstain the section.
2.12 Analysis of drug synergistic effect
Increasing concentrations of b-AP15 were co-treatment with IC(30) or IC(50) of imatinib (IM), the cell viability were measured by MTS assay. CompuSyn software was used to calculate the combination index (CI) which is a representative of the combined effects of two drugs.2122 The CI value less than, equal to, and greater than 1 indicates a synergistic, additive, and antagonistic effect, respectively.
2.13 Statistical analysis
Each experiment was repeated at least three times, and the graphs were all shown mean ± SD. p < .05 was considered statistically significant. Statistics were performed using Graphpad Prism 7.0 software. One-way ANOVA followed by Tukey's test was adopted for multiple groups comparison.
3 RESULTS
3.1 b-AP15 suppresses the deubiquitinase activity of USP14 and UCHL5 and induces cytotoxic effect in BCR-ABLWT and BCR-ABLT315I CML cells
To establish the clinical relevance of tumour proteasomal deubiquitinases expression in CML patients, the expression of proteasomal deubiquitinases (USP14, UCHL5 and POH1) was examined in peripheral blood mononuclear cells (PBMCs) from CML patients as well as that from healthy donors. We found that the mRNA and protein level of USP14 and UCHL5 in PBMCs from CML patients was significantly higher than that from healthy donors (Figure 1A and B). However, the expression level of POH1 in CML patients was similar to that in normal human (Figure 1A and B). In addition, we have tested the USP14 and UCHL5 protein level in BCR-ABLWT (KBM5 and BaF3) versus BCR-ABLT315I (KBM5-T315I and BaF3-T315I) cells. But there were no significant differences between BCR-ABLWT and BCR-ABLT315I CML cells (Figure S1A). b-AP15, an inhibitor of USP14 and UCHL5, has been reported to impair the function of UPS.10 Thus, we analysed the UPS inhibitory effects of b-AP15 in BCR-ABLWT (KBM5, K562, and BaF3) and BCR-ABLT315I (KBM5-T315I and BaF3-T315I) CML cells. As expected, b-AP15 increased the levels of ubiquitinated proteins and p27, a protein routinely degraded by the proteasome (Figure 1C). In addition, we investigated the effects of b-AP15 on the level of DVL2 protein, which is a well-established substrate of proteasomal deubiquitinase.23 As expected, the results showed that b-AP15 enhanced the level of DVL2 protein, indicating the suppression of proteasome function (Figure S1B). However, unlike the 20S proteasome inhibitor bortezomib, b-AP15 did not affect the C-, T-, and CT-like enzymatic activities of 20S proteasome (Figure 1D). Furthermore, we performed an in vitro deubiquitinase activity assay with HA-Ub-VS, which binds to deubiquitinase active sites. We found that b-AP15 decreased HA-Ub-VS labelled USP14 and UCHL5 (Figure 1E), indicating that b-AP15 inhibited the activities of proteasomal deubiquitinases in CML cells.
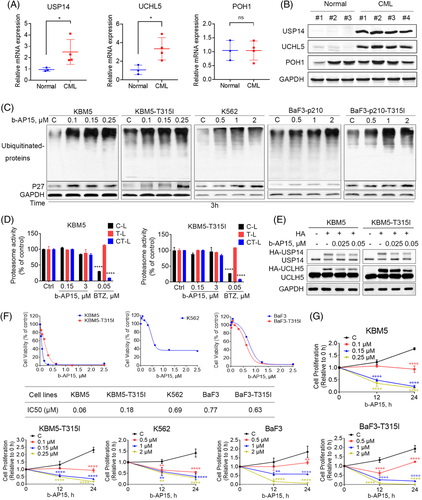
Next, the cytotoxic effect of b-AP15 was tested in a panel of TKI-sensitive and -resistant CML cells. We observed that b-AP15 suppressed the cell viability of BCR-ABLWT and BCR-ABLT315I CML cells (Figure 1F). Moreover, trypan blue dye exclusion assay showed that b-AP15 suppressed the cell proliferation of BCR-ABLWT and BCR-ABLT315I CML cells (Figure 1G). Collectively, these findings illustrate that inhibition of proteasomal deubiquitinases by b-AP15 kills TKI-sensitive and -resistant CML cells.
3.2 b-AP15 triggers caspase-dependent apoptosis in BCR-ABLWT and BCR-ABLT315I CML cells
To explore the mechanisms of b-AP15-induced cytotoxic effect, we performed the annexin V/propidium iodide assay to evaluate cell death. The results showed that b-AP15 induces cell apoptosis in BCR-ABLWT and BCR-ABLT315I CML cells (Figure 2A). Apoptosis is mainly mediated by caspase-mediated cleavage cascade.24 To verify whether b-AP15 activates the classical caspase signalling pathway, we examined the cleaved counterparts of caspase-3, -8, -9 in BCR-ABLWT and BCR-ABLT315I CML cells. We found that b-AP15 caused activation of caspases, as well as cleavage of PARP (Figure 2B and C). b-AP15 also caused a decrease in mitochondrial membrane potential (Figure 2D), which has been linked to the induction of apoptosis. Consistently, mitochondrial AIF and cytochrome C proteins were released into the cytosol in b-AP15-treated CML cells (Figure 2E), suggesting b-AP15 induced the opening of mitochondrial pore transition. Furthermore, b-AP15 induced downregulation of anti-apoptosis proteins, including Bcl-2, Mcl-1, and Bcl-xl in BCR-ABLWT and BCR-ABLT315I CML cells (Figure 2F). Likewise, b-AP15 induced the cleavage of XIAP, which may reflect the activation of caspases. These results strongly support that b-AP15 kills TKI-sensitive and -resistant CML cells via caspase-dependent apoptosis.

3.3 b-AP15 activates ER stress and suppresses BCR-ABL signallings in CML cells
To explore the relationship between proteasome inhibition and apoptosis, we investigated the markers of proteasome inhibition and cell apoptosis at sequential time points. Importantly, the accumulation of ubiquitinated proteins occurred significantly earlier than PARP cleavage in CML cells treated with b-AP15 (Figures 3A), suggesting that apoptosis is triggered after UPS inhibition by b-AP15. It has shown that dysfunction of UPS results in intracellular ER stress through the accumulation of misfolded proteins.25 Indeed, b-AP15 increased the expression of p-EIF2ɑ, ATF4 and CHOP, while it inhibited the expression of PERK (Figure 3B), which are hallmarks of ER stress. Moreover, we have detected ER stress at earlier time points and found that ER stress was activated at 0.5 h, a time point without visible apoptosis (PARP cleavage) (Figure S1C). Thus, ER stress is indeed a sensitive indicator of proteasome inhibition. Furthermore, we found that TUDCA (ER stress inhibitor) partially reduced b-AP15-induced apoptosis in KBM5 and KBM5-T315I cells (Figure S1D). These results demonstrate that b-AP15 triggers ER stress to activates cell apoptosis by proteasome inhibition.
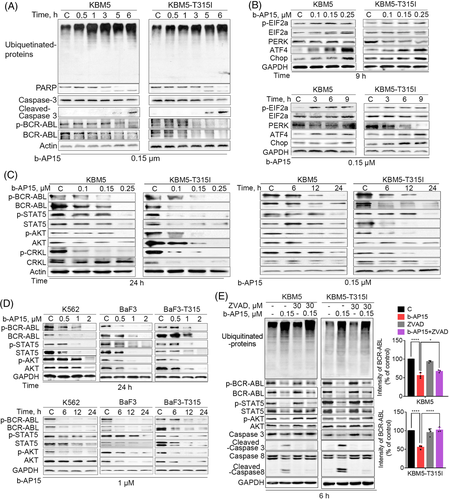
Given the key role of BCR-ABL in the malignant progression of CML, we tested the effect of b-AP15 on BCR-ABL-related signallings. As shown in Figure 3C and D, BCR-ABL and its phosphorylation was decreased in b-AP15-treated BCR-ABLWT and BCR-ABLT315I cells. Furthermore, the cascade signalling pathways of BCR-ABL, including STAT5, AKT and CRKL, were also suppressed by b-AP15 (Figure 3C and D). Next, we investigated how did b-AP15 induce downregulation of BCR-ABL. Our and other group have demonstrated that BCR-ABL can be cleaved by caspases.20, 26. Thus, we examined whether activation of the caspase pathway is responsible for b-AP15-induced downregulation of BCR-ABL. We observed that co-treatment with z-VAD-fmk (a caspase inhibitor) inhibited the downregulation of BCR-ABL and the cascade signalling pathways of BCR-ABL in CML cells following the treatment with b-AP15 (Figure 3E). However, there is no change in ubiquitinated proteins (Figure 3E), indicating that UPS inhibition may be an upstream event to the downregulation of BCR-ABL signallings. These results indicate that b-AP15-induced activation of caspases is partially essential for BCR-ABL downregulation in TKI-sensitive and -resistant CML cells.
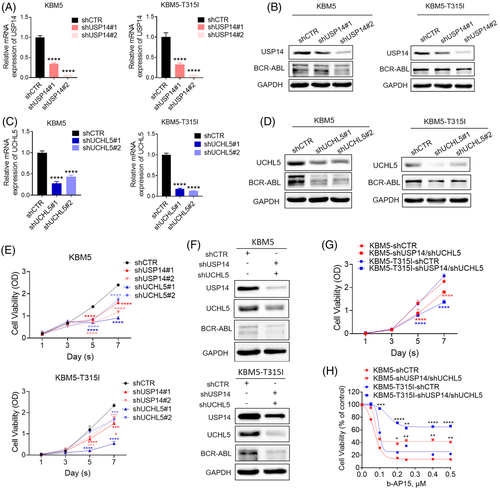
3.4 Pharmacological or genetic targeting of USP14 and UCHL5 induces cytotoxic effect in CML cells
To test whether USP14 and UCHL5 maintain cell proliferation of CML, we knocked down USP14 and/or UCHL5 in KBM5 and KBM5-T315I cells and observed that knockdown of either USP14 or USPL5 alone decreased BCR-ABL expression and CML cell viability (Figure 4A–E). Moreover, the combined knockdown of USP14 and UCHL5 induced a robust decrease in BCR-ABL protein level and cell viability of CML cells (Figure 4F and G). Importantly, the combined knockdown of USP14 and UCHL5 attenuated b-AP15-induced cytotoxic effect in CML cells (Figure 4H), further confirming that USP14 and UCHL5 indeed act as direct target of b-AP15. Consistently, Hinokitiol copper complex (HKCu), another reported USP14 and UCHL5 inhibitor,27 also decreased cell viability in CML cells (Figure S1E). Moreover, we overexpressed USP14 and/or UCHL5 in KBM5 and KBM5-T315I cells and found that overexpression of USP14 and/or UCHL5 inhibited the anti-proliferative effects of b-AP15 in KBM5 and KBM5-T315I cells (Figure S1F and G). In conclusion, these findings demonstrate that the inhibition of USP14 and UCHL5 may kill TKI-sensitive and -resistant CML cells through downregulating BCR-ABL levels.
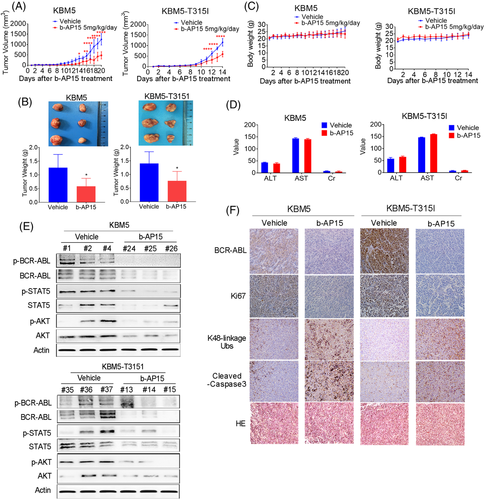
3.5 b-AP15 suppresses the growth of CML xenografts
To explore the antitumour effect of b-AP15 in vivo, KBM5 and KBM5-T315I cells were injected into the subcutis of nude mice. Every cohort was randomly separated into vehicle group and b-AP15 treatment group. Continuous b-AP15 treatment caused prolonged inhibition of tumour growth of CML xenografts (Figure 5A). Meanwhile, compared with the vehicle group, both tumour size and tumour weight were decreased in the b-AP15 group (Figure 5B). However, no significant difference was found in the body weight between two groups (Figure 5C). Likewise, the value of hepatorenal toxicity indicators (e.g. ALT, AST and Cr) were indistinguishable between two groups (Figure 5D). Therefore, b-AP15 appears to be safe and effective at the tested doses. Moreover, expression of BCR-ABL, STAT5, AKT and their phosphorylation counterparts were decreased by b-AP15 treatment (Figure 5E), indicating the tyrosine kinase cascade of BCR-ABL was suppressed by b-AP15. Also, the immunohistochemical staining of tumour sections showed an increase of K48-linkage ubiquitinated proteins and a decrease of BCR-ABL and Ki67 in the b-AP15 treatment group compared with the vehicle group (Figure 5F and Figure S1H). Moreover, the increased cleaved-caspase 3 also observed in IHC assay (Figure 5F). The results corroborate that b-AP15 exhibits potent in vivo efficacy with a favourable safety profile for the treatment of CML.
3.6 b-AP15 induces cell apoptosis in PBMCs from CML patients
To further verify the activity of b-AP15 in CML cells and provide research evidence for its clinical application, we explored the biological function of b-AP15 on PBMCs from BCR-ABLWT (#1-#7) and BCR-ABLMUT (#8 and #9) CML patients. The clinical and biological characteristics of CML samples collected in this study were shown in Table S1. In this regard, b-AP15 suppressed the cell viability of primary CML cells, with IC50 ranged from 0.1 to 1.49 µM (Figure 6A). Similar to the results of cultured cell lines, b-AP15 triggered cell apoptosis in these primary CML cells (Figure 6B and C). Moreover, b-AP15 induced cleavage of PARP and caspase 3, as well as downregulation of Mcl-1 and upregulation of Bax (Figure 6D), further supporting the anti-cancer activities of b-AP15 in CML. It worth noting that PBMCs from healthy donors was relatively tolerant to b-AP15, with IC50 of 5.8–10.4 µM, as we reported previously.28 These results indicate that b-AP15 may serve as an efficient and safe anticancer agent for CML patients.
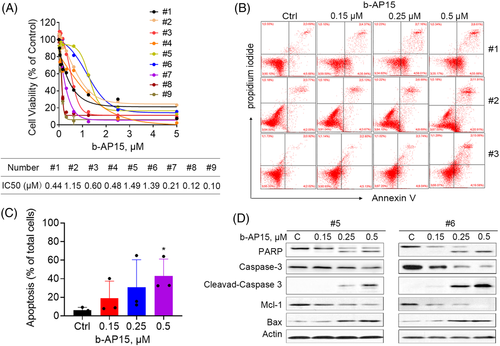
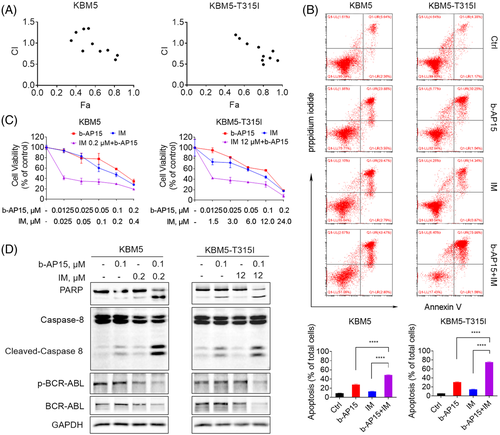
3.7 b-AP15 and imatinib synergistically induce cell apoptosis in BCR-ABLWT and BCR-ABLT315I cells
Since b-AP15 showed a significant reduction effect on BCR-ABL, we speculated a synergistic effect between tyrosine kinase inhibitor imatinib and proteasomal deubiquitinase inhibition. To verify this hypothesis, we performed MTS assay to examine the cell viability and calculated the combination index based on their concentration and biological activity. The results showed that the combination index values of imatinib and b-AP15 at most doses were lower than 1 in BCR-ABLWT and BCR-ABLT315I CML cells, indicating a synergism between imatinib and b-AP15 (Figure 7A). Imatinib in combination with b-AP15 dramatically reduced cell viability when compared with cells treated with either agent alone (Figure 7B). Similarly, knockdown of USP14 and UCHL5 enhanced imatinib reduced cell viability in CML cells (Figure S1I). Combined treatment with imatinib and b-AP15 also resulted in a synergistic apoptosis- inducing effect in KBM5 and KBM5-T315I cells (Figure 7C). Furthermore, cleaved caspase-8 and PARP proteins were increased in the combined group, while BCR-ABL protein and its phosphorylation were decreased (Figure 7D). These findings confirm the synergistic inhibition of imatinib and b-AP15 on TKI-sensitive and -resistant CML cells.
4 DISCUSSION
Proteasome tightly regulates the degradation of most intracellular proteins, which is a crucial mechanism to maintain cellular homeostasis. There are three proteasomal deubiquitinases USP14, UCHL5, and POH1, which function as a critical step regulates protein degradation through removing the poly-ubiquitin chain from substrates.6 Recent studies have reported that proteasomal deubiquitinases are highly expressed in various cancers, suggesting a potential antineoplastic function of their inhibitors.29-31 However, the function of proteasomal deubiquitinases in CML has rarely been reported.
The patients with BCR-ABL-positive CML can benefit from target therapy with TKI, however the toxicity and side effects, the drug off-target risks, the resistance, and the distinction of disease molecular profile are the limiting factors for TKIs.32 It is pretty important to find the etiological mechanism of TKI resistance. Here, we compared the expression of proteasomal deubiquitinases between healthy donors and CML patients. Both USP14 and UCHL5 were expressed higher in CML patients, suggesting that USP14 and UCHL5 could be a novel target for CML treatment. This was partially consistent with the report that USP14 was highly expressed in CML compared with normal marrow.33 Interestingly, several deubiquitinase, such as USP47, is transcriptionally regulated by BCR-ABL.33 However, whether BCR-ABL transcriptionally controls USP14 or UCHL5 in CML is still unknown. In addition, we demonstrated that knockdown of USP14 and UCHL5 increased imatinib sensitivity and cell apoptosis in BCR-ABLWT and BCR-ABLT315I CML cells. These results open up alternative avenues for overcoming imatinib resistance, such as using USP14 and UCHL5 inhibitors as single agents or combined with imatinib in the treatment of T315I mutant CML patients.
b-AP15 contains an α,β-unsaturated carbonyl, which reversibly reacts with cysteine proteases USP14 and UCHL5, but not decreases the activities of the 20S proteasome.34 It has shown that b-AP15 rapidly distributes into cells and elicits rapid apoptosis.34 We observed that b-AP15 showed similar effects through targeting USP14 and UCHL5. Moreover, our results also demonstrated that b-AP15 suppressed cell growth through downregulating the expression of BCR-ABL in CML cells and CML xenografts. Importantly, b-AP15 was toxic to PBMCs from CML patients and synergistically induced cell apoptosis with imatinib on BCR-ABLWT and BCR-ABLT315I CML cells. Noteworthy, VLX1570, an analogue of b-AP15, was advanced into Phase I clinical trial, although this project was discontinued due to pulmonary toxicity.35 It has been postulated that the adverse events appear to be related to the formulation.35 Nevertheless, efforts directed at identifying proteasome deubiquitinase inhibitors carries important clinical potential.
It has been documented that the accumulation of excessive ubiquitinated proteins activates ER stress and ultimately elicits cell death.36, 37 Recent studies have reported that b-AP15 and its analogue triggered cell apoptosis via activation of ER stress in leukaemia and solid tumour cells.38-40 In agreement with these findings, our work revealed that b-AP15 treatment induced ER stress and cell apoptosis in BCR-ABLWT and BCR-ABLT315I CML cells, which may be related to the accumulation of unfolded protein response by inhibiting USP14 and UCHL5. In addition, BCR-ABL can prevent apoptotic death in CML cells partly by inducing Bcl-2 and MCl-1 expression.41, 42 Our work revealed that b-AP15 decreased the expression of anti-apoptosis proteins including Bcl-2 and Mcl-1, which may contribute to b-AP15-induced cell apoptosis in CML.
It is worth noting that BCR-ABL was slightly decreased by b-AP15 stimulation at an earlier time before the presence of cell apoptosis, and it was obviously decreased
at a later time point accompanied by a strong induction of cell death. This relationship indicates that downregulation of BCR-ABL may be related to the occurrence of apoptosis. Our data demonstrated that BCR-ABL downregulation partially relied on the activation of caspases, which directly mediated the cleavage of BCR-ABL and the proteins of key downstream signalling pathway regulated by BCR-ABL, such as STAT5, p-STAT5, AKT, and p-AKT in the same condition. However, other caspase-independent mechanism may also be involved, which have not yet been elucidated. Recent studies have confirmed that BCR-ABL can be degraded via the UPS, and several deubiquitinases have been reported to participate in this process. For instance, USP7 and USP25 cleave K48-linkage poly-ubiquitination conjugate from BCR-ABL, which accelerates BCR-ABL degradation through UPS.43, 44 In addition, inhibition of USP9X triggers K63-linkage poly-ubiquitination of BCR-ABL, resulting in its accumulation in aggresomes.45 It is still unknown whether USP14 or UCHL5 regulates the deubiquitination and subsequent degradation of BCR-ABL protein. Therefore, further interrogation of the mechanism involved in BCR-ABL degradation is required before firm conclusions can be drawn.
In conclusion, this study demonstrates that b-AP15 induces cell apoptosis and downregulates BCR-ABL in BCR-ABLWT and BCR-ABLT315I CML cells (Figure 8). These findings provide a novel understanding of USP14 and UCHL5 as an important therapeutic target for TKI-resistant CML treatment.
ACKNOWLEDGEMENTS
We are thankful to all participants for their valuable contributions. This work was supported by NSFC (82170177/H0809, 81670154/H0812), Project (2020KTSCX10) from the Foundation of Innovation Projects of General Colleges and Universities in Guangdong Province, GD-NSF (2021A1515011334) and Key Discipline of Guangzhou Education Bureau (Basic Medicine) (201851839), and the project (202201010811) from the Foundation of Guangzhou Science and Technology Innovation Committee to Xianping Shi. This work also supported by China Postdoctoral Science Foundation (2021M690791) to Liling Jiang.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.




