MTHFR inhibits TRC8-mediated HMOX1 ubiquitination and regulates ferroptosis in ovarian cancer
Xiang Wang and Zhijie Xu made equal contributions to this work.
Dear Editor,
The study is the first to confirm that methylenetetrahydrofolate reductase (MTHFR) could inhibit HMOX1 ubiquitination degradation by competitive interaction with TRC8, followed by blocking the occurrence of ferroptosis in ovarian cancer (OV) cells, and promote the tumour cells growth. OV attributes to the world's second most familiar cause of gynaecologic cancer death.1 Recent research studies identified that the polymorphisms of MTHFR correlate with the risk of common gynaecological cancers.2, 3 However, there is a dearth of studies with in-detail elucidation of functions and mechanisms of MTHFR in OV.
MTHFR is a key enzyme involved in folic acid metabolism.2 In this study, it was found that MTHFR was upregulated in OV cell lines (SKOV3, TOV112D, A2780 and OVCAR3) and tissues both in mRNA and protein levels (Figures 1A,B and S1A). The mRNA and protein expression of MTHFR was found to be higher in A2780 and OVCAR3 cells, which led us to the selection of A2780 and OVCAR3 cells for further study. The higher expression of MTHFR was associated with poor overall survival (OS), progression-free survival (PFS) and post-progression survival (Figure S1B–D). As shown in Figures 1C–F and S2A–C, it was found that the colony numbers and cell viability were obviously decreased in MTFHR-interfering cells compared to the control cells, as determined by the colony formation and CCK8 assays, respectively. In addition, we applied a lentivirus-medicated expression system to stably overexpress MTHFR in MTFHR-interfered OV cells (Figures 1G and S2D). By colony formation and CCK8 assays, the overexpression of MTHFR significantly reversed the inhibition effects of MTHFR knock-down in OV cells (Figures 1H–J and S2E,F). Cisplatin, cisplatinum or cis-diamminedichloroplatinum(II) (CDDP) are a classical antitumour drug, which could be considered an inducer for ferroptosis.4, 5 Moreover, the expression of MTHFR in OV cells was decreased in a dose-dependent manner of CDDP (Figure S3A,B). Interestingly, the concentration gradient-based treatment with CDDP, MTHFR downregulation could inhibit the colony formation ability and viability in OV cells (Figure S3C–F). Additionally, an overexpressed level of MTHFR significantly reversed the effect of MTHFR knock-down in MTHFR-interfered OV cells under CDDP treatment (Figure S3G–J). Therefore, it is apparent to examine the relationship between MTHFR and drug sensitivity, and their contribution towards the therapeutics of OV.
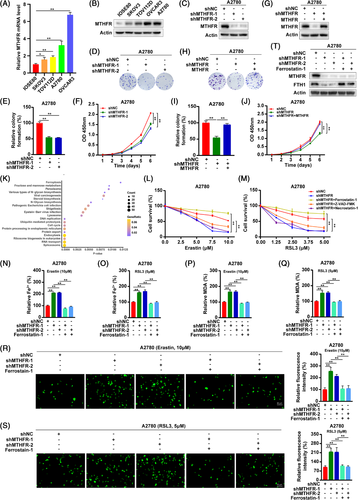
As shown in Table S1, total 2040 proteins, obtained from mass spectrometry, were differentially expressed after MTHFR knock-down compared to the control group, including HMOX1. The KEGG functional enrichment analyses suggested that differentially expressed proteins were significantly related to ferroptosis through the LinkInterpreter module (Figure 1K). Ferroptosis is an iron-dependent regulated cell death induced by lipid peroxidation.6 In addition, the model based on ferroptosis related-genes was contribute to predict the prognosis of OV patients and induction of ferroptosis could enhance the inhibitory effect of CDDP on OV cells.7, 8 To ascertain this result, we examined the viability of OV cells under the treatment of ferroptosis inducers, erastin and RSL3. As shown in Figures 1L,M and S4A,B, the knock-down of MTHFR increased erastin- or RSL3-induced growth suppression in OV cells and ferroptosis inhibitor (ferrostatin-1) could significantly reverse the growth suppression of MTHFR knock-down. To further validate this observation, the OV cells were treated with erastin or RSL3, which could increase the Fe2+, lipid peroxidation products (MDA) and ROS levels, whereas these effects could be reversed by ferrostatin-1 in OV cells (Figure S4C–J). Moreover, the knock-down of MTHFR increased the accumulation of Fe2+, MDA and ROS and decreased the protein level of FTH1 under erastin or RSL3 treatment and could be reversed by ferrostatin-1 in OV cells (Figures 1N–T and S5A–I). The overexpression of MTHFR significantly reversed the promotion effects of MTHFR knock-down on the accumulation of Fe2+ and MDA in OV cells (Figure S5J–Q). Anti-folate and anti-methionine strategies have been addressed extensively, whereas the clinical benefits of these approaches were inconclusive. This finding provided new ideas and rationality for clinical potential of MTHFR-based targeting therapy of ovarian cancer.
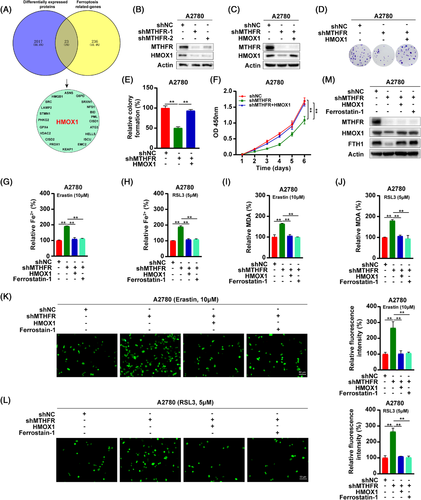
HMOX1 is a rate-limiting enzyme that degrade the haem into Fe2+.9 HMOX1 had a dual role in the ferroptosis regulation.10 This dual mechanism of HMOX1 was already present at early 1999.11, 12 Excessive activation of HMOX1 could induce ferroptosis in cancer cells.13 However, HMOX1 can degrade the haem into Fe2+.9 However, HMOX1 decreases the bioavailability of haem and plays an important anti-oxidative role in cancer cells.14 In a renal ischaemia-reperfusion model, the upregulation of HMOX1 could inhibit ferroptosis of renal tissues.15 In addition, knock-down HMOX1 in hepatocellular carcinoma cells could promote ferroptosis of cells.16 Nishizawa et al. also demonstrated that HMOX1 acted as an inhibitor of ferroptosis.17 The generation of Fe2+ promotes the synthesis of ferritin, which inhibits the occurrence of ferroptosis.10 To identify the downstream of MTHFR, we overlapped the ferroptosis related-genes and differentially expressed proteins after MTHFR knock-down and found HMOX1 was significantly downregulated18 (Figure 2A). Consisting with the mass spectrometry, HMOX1 was found significantly reduced in MTHFR-deficient OV cells (Figures 2B and S6A). Moreover, the overexpression of HMOX1 in MTHFR-deficient cells could not rescue the downregulated expression of MTHFR by shRNA, inferring that MTHFR regulated the ferroptosis of OV cells through influencing the expression of HMOX1 (Figures 2C and S6B). Then, colony formation and CCK8 experiments showed that the overexpression of HMOX1 significantly rescued the inhibition effect of MTHFR knock-down (Figures 2D–F and S6C–E). Moreover, treatment with CDDP downregulated MTHFR and HMOX1 expression in a dose-dependent manner in OV cells (Figure S6F–G). In MTHFR-deficient OV cells, the overexpression of HMOX1, significantly reversed the enhanced sensitivity to CDDP in OV cells by MTHFR knock-down (Figure S6H–M). Moreover, the overexpression of HMOX1 could significantly rescue the induced ferroptosis and downregulation of FTH1 in MTHFR-deficient OV cells (Figures 2G–M and S6N–V).
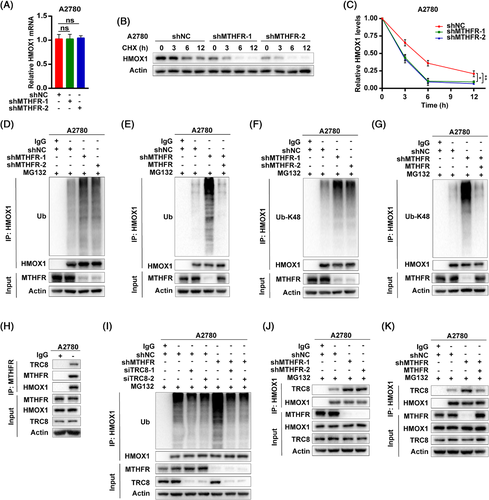
Mechanically, nuclear factor erythroid 2 related factor 2 (NRF2) has been reported to regulate HMOX1 expression by transcriptional modification.19, 20 In renal carcinoma cells, HMOX1 was found to be degraded through ubiquitination by E3-ligase TRC8, which resulted in the inhibition of cancer cell growth and migration.21 As MTHFR knock-down did not influence HMOX1 transcription level (Figures 3A and S7A), as well as NRF2 expression (Figure S7B,C), it is apparent that MTHFR mediated HMOX1 upregulation might be through post-translational modification. The cycloheximide chase assay revealed MTHFR knock-down significantly decreased protein stability of HMOX1 in A2780 and OVCAR3 cells (Figures 3B,C and S7D). Furthermore, the knock-down of MTHFR, increased ubiquitination especially K48-linked but not K63-linked polyubiquitination of HMOX1, whereas the overexpression of MTHFR could reverse this induction in MTHFR-deficient OV cells (Figures 3D–G and S7E–K). In addition, Co-IP showed MTHFR could form a complex both with HMOX1 and TRC8 in OV cells (Figures 3H and S7L). Interfered TRC8 by siRNA could abolish the increased ubiquitination of HMOX1 by MTHFR knock-down in OV cells (Figures 3I and S7M). The knock-down of MTHFR promoted the combination of TRC8 and HMOX1 (Figures 3J and S7N), whereas the overexpression of MTHFR reduced this integration in MTHFR-deficient OV cells (Figures 3K and S7O).
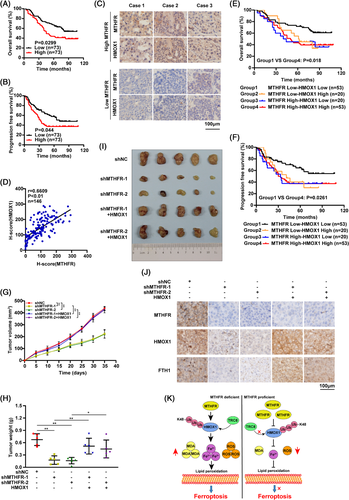
Using tissue microarray, high levels of MTHFR and HMOX1 were observed in OV patients, which was correlated with the poor prognosis (Figures 4A,B and S8A–C). Moreover, pathology grade, Ki67 and EGFR were found to be positively correlated with MTHFR in OV patients significantly (Table 1). Additionally, positive correlation was found between MTHFR and HMOX1 expression in OV patients (Figure 4C,D). The OS and PFS of double positive OV patients, harboured both higher expression of MTHFR and HMOX1, were significantly poorer than that of double negative OV patients (Figure 4E,F). Moreover, xenograft tumour model showed that MTHFR downregulation apparently delayed tumour weights and volumes, whereas this effect could be rescued by HMOX1 overexpression (Figure 4G–I). The expression of MTHFR, HMOX1 and FTH1 in tissues was confirmed by IHC (Figure 4J).
| MTHFR expression | |||||
|---|---|---|---|---|---|
| Characteristic | Total | Low (n = 53) | High (n = 54) | χ2 | P |
| Age (years) | .010 | .919 | |||
| ≥50 | 51 | 25 (49%) | 26 (51%) | ||
| <50 | 56 | 28 (50%) | 28 (50%) | ||
| Pathology grade | 4.457 | .035* | |||
| I–II | 18 | 13 (72%) | 5 (28%) | ||
| III | 89 | 40 (45%) | 49 (55%) | ||
| Tumour size (cm) | .895 | .344 | |||
| ≥10 | 70 | 37 (53%) | 33 (47%) | ||
| <10 | 37 | 16 (43%) | 21 (57%) | ||
| T | 5.616 | .018* | |||
| T1–T2 | 24 | 17 (71%) | 7 (29%) | ||
| T3 | 83 | 36 (43%) | 47 (57%) | ||
| N | .019 | .890 | |||
| N0 | 72 | 36 (50%) | 36 (50%) | ||
| N1 | 35 | 17 (50%) | 18 (50%) | ||
| M | .641 | .423 | |||
| M0 | 77 | 40 (49%) | 37 (51%) | ||
| M1 | 30 | 13 (43%) | 17 (57%) | ||
| Relapse | .853 | .356 | |||
| Yes | 94 | 45 (48%) | 49 (52%) | ||
| No | 13 | 8 (62%) | 5 (38%) | ||
| Metastasis | .976 | .323 | |||
| Yes | 11 | 7 (64%) | 4 (36%) | ||
| No | 96 | 46 (48%) | 50 (52%) | ||
| Ki67 | 5.490 | .019* | |||
| ≤.4 | 85 | 47 (55%) | 38 (45%) | ||
| >.4 | 22 | 6 (27%) | 16 (73%) | ||
| EGFR | 4.042 | .044* | |||
| ≤.75 | 73 | 41 (56%) | 32 (44%) | ||
| >.75 | 34 | 12 (35%) | 22 (65%) | ||
| PD-L1 | 1.183 | .277 | |||
| ≤.01 | 61 | 33 (54%) | 28 (46%) | ||
| >.01 | 46 | 20 (43%) | 26 (57%) | ||
- * P < 0.05 was considered significant.
In conclusion, our study demonstrated that MTHFR was upregulated in OV and the knock-down of MTHFR inhibited the growth of OV cells both in vitro and in vivo. Moreover, MTHFR could suppress the ferroptosis through blocking K48-linked ubiquitination of HMOX1 to stable HMOX1 (Figure 4K). Additionally, the high expression level of MTHFR was significantly associated with the poor prognosis of OV patients. In future work, it will be crucial to screen the potential role of MTHFR in cancer cells, which would pave the way for developing novel therapeutic drugs targeting MTHFR for diagnosis and therapeutic intervention of OV patients.
ACKNOWLEDGEMENTS
This study is supported by grants from the Science and Technology Innovation Program of Hunan Province (2021RC3029), the China Postdoctoral Science Foundation (2021T140754, 2020M672521) and the Postdoctoral Science Foundation of Central South University (248485). We thank Dr. Abhimanyu Thakur for assistance with the language editing.
CONFLICT OF INTEREST
The authors declare no conflict of interest.




