Phosphatidylethanolamine: A key player in lung disease
Abstract
Phosphatidylethanolamine (PE) is a pivotal glycerophospholipid that constitutes a significant portion of cellular membranes, playing a crucial role in maintaining membrane fluidity, supporting protein integration, and mediating signal transduction. In the lungs, PE is also a key component of pulmonary surfactant, which is essential for preserving alveolar stability and facilitating efficient gas exchange. Recent research has highlighted the association between dysregulated PE metabolism and various lung diseases, such as asthma, pulmonary fibrosis and chronic obstructive pulmonary disease. Nevertheless, the molecular mechanisms underlying these associations remain poorly understood, and the potential of PE as a therapeutic target or biomarker for lung diseases has yet to be fully explored. This review aims to provide a comprehensive overview of the biological functions and biosynthetic pathways of PE, with a particular focus on its roles in pulmonary physiology and pathology. We summarise current findings on PE alterations in different lung diseases and discuss the potential implications of targeting PE metabolism for therapeutic interventions.
-
Implication of PE in the aetiology of diverse diseases.
-
The pivotal role of PE in driving lipid peroxidation during ferroptosis.
-
Implications of PE metabolic dysregulation in pulmonary epithelial cells.
1 INTRODUCTION
Phosphatidylethanolamine (PE) is a glycerophospholipid containing an ethanolamine phosphate head group and is one of the main components of cellular membranes. It is the second most abundant phospholipid after phosphatidylcholine (PC), and together, they constitute more than half of the total membrane phospholipid content.1 PE plays a key role in regulating membrane architecture and dynamics. By modulating membrane fluidity through its interactions with proteins and altering the physical state of the lipid bilayer, PE influences numerous cellular processes such as signalling, vesicular trafficking, and molecular transport.2 Due to its relatively smaller polar head group compared to its fatty acid chains, PE exhibits limited bilayer-forming capacity and instead tends to form hexagonal phases within membranes. This propensity facilitates membrane fusion and fission, aids in protein integration into membranes, and triggers conformational shifts in protein structures.3, 4 Beyond its structural roles, PE is also linked to protein synthesis and function, supports energy production through oxidative phosphorylation, and plays a key role in autophagy and mitochondrial stability. Additionally, it serves as a precursor for other lipid synthesis.5 PE is closely related to the function of intracellular organelles such as lipid droplets, the endoplasmic reticulum, and mitochondria, all of which are important in cellular metabolism and stress responses.6, 7 Moreover, PE participates in signal transduction pathways, including the ‘mesenchymal-to-epithelial transition’, a key event in the early stages of reprogramming and differentiation.8
Pulmonary surfactant, synthesised and secreted by alveolar type II epithelial cells, is composed of lipids (∼90%) and proteins (∼10%). PE represents a significant fraction of lipid constituents. In the alveoli, PE contributes to reducing surface tension at the alveolar air-liquid interface, thus maintaining alveolar stability, preventing alveoli from atrophying after expiration, and ensuring efficient gas exchange.9 An increasing number of studies suggest that metabolic abnormalities in PE are associated with the pathogenesis of a variety of lung diseases. For example, PE attenuates bleomycin-induced pulmonary fibrosis.10 In individuals with asthma, particularly those with eosinophilic asthma, PE levels are elevated compared to those in non-eosinophilic asthma, and these levels also show some correlation with lung function indicators.11 This review summarises the dual roles of PE in maintaining physiological homeostasis and contributing to pulmonary pathogenesis. We specifically discuss the functional divergence among different PE molecular species and evaluate their biomarker potential for disease stratification. Elucidating the regulatory mechanisms governing PE metabolism is critical for the development of targeted therapeutic interventions.
1.1 Biological functions of PE
PE is a key component of cell membranes and serves multiple fundamental biological functions. In the liver, PE acts as a substrate for the liver enzyme PE N-methyltransferase, which synthesises approximately one-third of the total PC.12, 13 Additionally, PE acts as a precursor in the production of anandamide, a ligand that activates cannabinoid receptors in the brain and is implicated in numerous neurophysiological processes.14, 15 Furthermore, PE serves as the donor of the ethanolamine group, a critical component in the glycosylphosphatidylinositol anchoring of proteins. This process is essential for the proper functioning and localisation of certain proteins within the cell membrane.16 Remarkably, PE has also been recognised as the only intrinsic component in the brain necessary for prion infectivity and facilitating its transmission in mammals.17
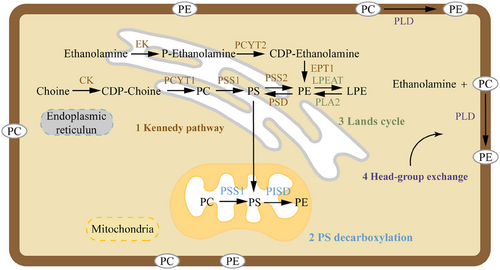
The contribution of each PE biosynthetic pathway varies depending on the cell type. In mammalian cells, PE is generated through four distinct biosynthetic pathways, although two of them contribute minimally under most physiological conditions (Figure 1). The primary pathways include: (i) the Kennedy pathway, also known as the CDP-ethanolamine pathway, which culminates on endoplasmic reticulum membranes, and (ii) the pathway involving phosphatidylserine (PS) decarboxylation, which occurs specifically at the inner mitochondrial membranes. A third pathway, prevalent in yeast and potentially significant in mammals, involves the acylation of lyso-PE. An alternative pathway for PE biosynthesis exists in the ER, involving calcium-mediated base exchange of PS with ethanolamine; however, this mechanism contributes minimally to overall PE production in mammalian systems.18 The relative activity of these pathways in different cell types may vary depending on cellular demands and metabolic state. Phosphate cytidylyltransferase 2 (Pcyt2) functions as the key regulatory enzyme in PE biosynthesis via the Kennedy pathway. Deficiency in Pcyt2 impairs PE-dependent lipidation of microtubule-associated protein 1 light chain 3 (LC3)-II during autophagy, thereby blocking autophagosome maturation.19 Studies have demonstrated that Pcyt2 operates through multilevel regulatory mechanisms and is indispensable for embryonic development, as systemic Pcyt2-knockout mice exhibit embryonic lethality before day 8.5 post-coitum.20 Maternal asthma elevates PE levels in amniotic fluid, predominantly in oxidised species, which potentially enhances inflammatory mediator production. This establishes a pro-inflammatory fetal microenvironment that may predispose offspring to heightened allergic responses and exacerbate postnatal inflammation. Such conditions likely impair fetal lung development.21 PE derivatives demonstrate biomarker potential for assessing fetal growth and lung health in amniotic fluid, though causal pathological impacts require further elucidation. Recent researches underscore the therapeutic potential of inhibiting enzymes involved in PE biosynthesis in oncology. Specifically, targeting the Kennedy pathway, a primary route for PE production, could be particularly effective. This suggests that disruption of this pathway could suppress cancer cell growth and survival, offering a promising strategy in cancer chemotherapy.22
1.2 Association of PE and pulmonary diseases
Studies have shown that changes in PE levels are associated with inflammatory responses, alveolar epithelial cell damage, and the progression of pulmonary fibrosis. PE plays a multifaceted role in maintaining pulmonary homeostasis, not only as a structural membrane component but also as a regulator of critical cellular processes such as autophagy (Figure 2) and surfactant function.
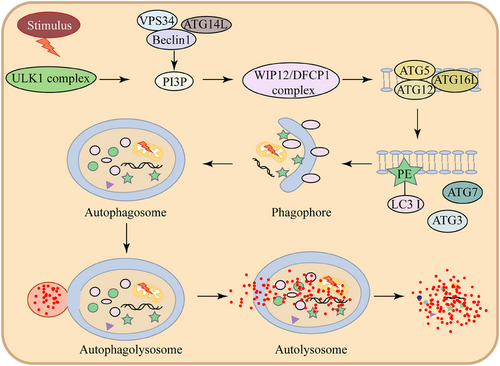
A notable example is Legionella pneumophila, an intracellular bacterium that circumvents the host's autophagy defence by introducing the effector protein RavZ (Figure 3). RavZ specifically detaches LC3 proteins from PE on autophagosomal membranes,23 thereby disrupting autophagy initiation. The conversion of soluble LC3 to membrane-anchored LC3-PE (LC3-II) represents a critical step in the LC3 conjugation pathway, playing an indispensable role in autophagy. This post-translational modification regulates fundamental autophagic processes, from autophagosome biogenesis and cargo recognition to autophagosome-lysosome fusion.24 Pulmonary surfactant consists of a blend of 10% specialised surfactant proteins, namely SP-A, SP-B, SP-C, and SP-D, along with 90% lipids, predominantly phospholipids.25 Although PE comprises only about 1.5% of surfactant phospholipids, its conical molecular structure, resulting from a small polar head group, imparts biophysical properties that support negative membrane curvature and lateral pressure modulation. Beyond its structural roles, PE exerts anti-fibrotic effects by binding to putative cell surface receptors on lung fibroblasts, thereby activating a phospholipase C-dependent signalling cascade.26 This activation triggers inositol trisphosphate (IP3)-mediated calcium (Ca2+) release from the ER, followed by sustained Ca2+ influx via store-operated calcium entry. The resulting elevation in intracellular Ca2+ levels drives a dual anti-fibrotic response: (1) suppression of collagen type I gene transcription through Ca2+-dependent transcriptional regulators,27 and (2) induction of caspase-3/7-dependent apoptosis. It has been shown to have a potential therapeutic role for PE in mitigating pulmonary fibrosis.
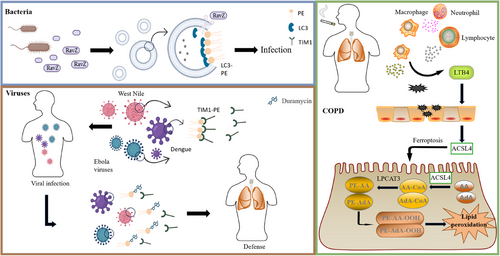
The PS receptor contributes to two key biological processes: the phagocytic removal of apoptotic cells and the cellular internalisation of numerous enveloped viral particles. Among these receptors, T-cell immunoglobulin and mucin-domain protein 1 (TIM1) has been shown to bind not only PS but also PE (Figure 3). TIM1 exhibits high-affinity binding to PE, a molecular interaction that mediates two distinct biological functions: (i) the efficient phagocytic clearance of apoptotic cells, and (ii) the enhancement of cellular entry for multiple pathogenic viruses spanning the Flaviviridae (e.g. West Nile virus and dengue virus) and Filoviridae (e.g. Ebola virus) families.28 Further mechanistic investigations have revealed that duramycin, a PE-specific cyclic peptide antibiotic, can effectively inhibit host cell invasion by these viruses through selectively blocking the TIM1-mediated viral endocytic pathway,28 thereby effectively inhibiting the invasion of host cells by these viruses. These findings imply that interfering with the interaction between PE and PS receptors could be a potential broad-spectrum antiviral approach. During the mid-to-late stages of infection, the levels of some PEs increase.29 In sputum metabolomics studies of chronic obstructive pulmonary disease (COPD) patients, significant changes in some metabolites were observed in COPD patients compared to healthy individuals, including PE.30 Specifically, among 24 COPD-depleted modules depleted in COPD, 275 metabolites were identified, of which 89 were PCs or PEs. This finding aligns with the catabolism of these phospholipids observed in the COPD metagenomes. A lipidomic study revealed that the levels of PE 42:7, PE 37:3, PE 35:1 and PE 39:6 were specifically elevated in severe acute pneumonia, but not in acute pulmonary embolism or acute exacerbations of COPD.31
Studies have demonstrated that PE can participate in biological processes such as ferroptosis, immune response28 and neurotransmitter synthesis. During ferroptosis, PEs esterified with polyunsaturated fatty acids—specifically arachidonic acid (AA) or adrenic acid (AdA)—are selectively enriched through acyl-CoA synthetase long-chain family member 4 (ACSL4)-mediated esterification and lysophosphatidylcholine acyltransferase 3 (LPCAT3)-dependent membrane remodelling.32 These PE species are then oxidised by 15-lipoxygenase (15-LOX) within ER-associated compartments, where their non-bilayer hexagonal phase conformation facilitates enzymatic access. This process generates doubly or triply oxygenated 15-hydroperoxy-PE (PE-OOH) species, which act as direct execution signals of ferroptosis. When glutathione peroxidase 4 (GPX4) is inactivated or depleted, PE-OOH accumulation escalates due to failed reduction to non-toxic PE-OH derivatives. The resulting unresolved lipid peroxidation propagates membrane damage through reactive electrophilic mediators, ultimately executing iron-dependent cell death.33 In this study, four PE oxygenates (PE-(C18:0/C20:4)+2[O], PE-(C18:0/C22:4)+2[O], PE-(C18:0/C20:4)+3[O], PE-(C18:0/C22:4)+3[O]) were screened as markers of ferroptosis. However, phospholipids (PLs) esterified with fatty acids with varying degrees of saturation exhibit distinct effects on ferroptosis. For instance, ACSL3 confers ferroptosis resistance by activating exogenous monounsaturated fatty acids (MUFAs) to MUFA-CoAs, which are preferentially incorporated into membrane phospholipids, displacing oxidatively vulnerable PUFA-PLs. This lipid remodelling diminishes the pool of peroxidation-susceptible PUFAs at the plasma membrane, thereby attenuating iron-dependent lethal lipid reactive oxygen species accumulation at this subcellular compartment. Consequently, ACSL3-dependent MUFA incorporation establishes a ferroptosis-resistant cellular state characterised by diminished plasma membrane oxidizability.34 Although this study examined PLs collectively without specific isolation of PEs, it is plausible to speculate that PEs—as critical oxidizable components of PLs—contribute centrally to maintaining the observed effects. This hypothesis warrants further experimental confirmation in future studies. Notably, dysregulated PE metabolism has been shown to affect B-cell follicular recruitment and follicular helper T-cell differentiation, thereby interfering with humoral immune responses to organismal infection.35
By regulating the PE metabolic pathway, it can affect cellular energy metabolism and lipid metabolism, and thus have an impact on the development of diverse disorders spanning cardiovascular (atherosclerosis), metabolic (insulin resistance and obesity)36 and respiratory systems. Phospholipid-monounsaturated fatty acyl species, including PE-monounsaturated fatty acyl, serve as potent inhibitors of ferroptosis, primarily by reducing the accumulation of lipid peroxides in cells.34 In contrast, recent studies have shown that ACSL4-mediated synthesis of PE-polyunsaturated fatty acyl is closely related to cellular metabolic adaptability and contributes to cancer cell metastasis.37 This finding lays the foundation for the development of novel drugs that target tumour metastasis. This also provides new research directions for diseases related to PE metabolic disorders, including those affecting the lungs. However, the precise molecular mechanisms by which different acyl side chains influence PE function remain incompletely understood and require further exploration. In COPD patients, cigarette smoke exposure leads to the recruitment of macrophages, neutrophils, and lymphocytes to small airways and lung parenchyma. These infiltrating immune cells secrete inflammatory factors that induce oxidative stress and the death of pulmonary epithelial cells.38 Specifically, the accumulation of monocyte-derived macrophages increases leukotriene B4 secretion and triggers epithelial cells to express ACSL4, thereby inducing ferroptosis in alveolar type 2 epithelial cells.39
2 CONCLUSION
This review systematically examines the multifaceted biological functions of PE and its mechanistic involvement in lung health and disease, including asthma, pulmonary fibrosis, and COPD. It highlights the importance of PE in maintaining cellular functions and its involvement in various pathological processes. Recent studies have shown that specific changes in PE levels can be detected in the early stages of lung diseases such as asthma and COPD. These changes may serve as potential biomarkers for early diagnosis, thereby enabling more timely and effective clinical interventions. For example, elevated levels of certain PE species in sputum or blood samples have been associated with eosinophilic asthma, offering potential for early diagnosis before the onset of severe clinical symptoms.30 Moreover, PE is a key component of autophagosomes, and its levels can influence the efficiency of autophagy. During pulmonary fibrosis development, PE-mediated autophagy defects result in failed clearance of damaged cellular elements, initiating a cascade of tissue damage.24 These results indicate that PE metabolism represents a promising therapeutic target for lung and metabolic disorders. Nevertheless, additional investigations are necessary to comprehensively decipher the molecular mechanisms underlying PE dysregulation. What are the specific functional differences of PE with varying acyl chain lengths and saturation levels in specific lung cells, such as alveolar epithelial cells, macrophages and fibroblasts? Furthermore, do these different PE molecular species have distinct mechanisms of action across various pulmonary diseases (e.g. COPD, idiopathic pulmonary fibrosis, asthma and lung cancer), and how are they precisely regulated? With recent advances in research technologies, isotopic tracing of the real-time kinetics of PE synthesis, oxidation, and degradation has become increasingly feasible. Leveraging such tools to construct a comprehensive metabolic map of PE based on such approaches may enable targeted interventions at critical regulatory nodes to modify disease progression, particularly in the context of pulmonary disorders.
AUTHOR CONTRIBUTIONS
Linlin Zhang contributed to the preparation and collection of original literature and figures, and the writing; Wanxin Duan and Liyang Li were responsible for the scientific quality and editing of the manuscript.
ACKNOWLEDGEMENTS
This work was supported by the National Natural Science Foundation of China (82400038 and 82300048), China Postdoctoral Science Foundation (2024M750548 and 2023M730661) and Open Project of Fujian Key Laboratory of Lung Stem Cells (FGXB202401).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
Not applicable
Open Research
DATA AVAILABILITY STATEMENT
Not applicable




