Marine toxin (+)-chaetocin-induced apoptosis of lung large cell carcinoma cell lines through cell cycle arrest via CDKN1A expression and replicative stress
Abstract
Lung large cell carcinoma is a common type of lung cancer with poor prognosis. Although targeted drugs have achieved enormous success in treating non-small-cell lung carcinoma (NSCLC), new chemotherapy is needed since the emerged drug resistance always hinders curative effects. The fungal toxin (+)-chaetocin demonstrated strong antineoplastic activities against the tested lung large cell carcinoma cell lines H460 and H661 at submicromolar concentrations. Further research demonstrated that (+)-chaetocin effectively induced H460 apoptosis at 10–30 nM concentrations, while cell death occurred at 60 nM concentrations due to DNA duplication errors. Cell cycle and transcriptional analyses proved that cell cycle arrest via CDKN1A expression and the comprehensive replicative stress of (+)-chaetocin are key factors in the (+)-chaetocin working mechanisms.
1 INTRODUCTION
Marine secondary metabolites are precious natural sources that produce chemical novelty with promising antineoplastic activities.1, 2 As important landmarks of marine drug research, the successes of eribulin (E7389) and E7130, both of which were developed from sponge-sourced halicondrin series toxins and underwent severe tests under the great guidance of Prof. Y. Kishi at Harvard University, encouraged scientists worldwide (Figure 1A).3, 4 Among these abundant marine secondary metabolites, (+)-chaetocin is a well-known dimer member of the 3,6-epidithio-diketopiperazine class of marine fungal natural molecules, which was first discovered from the marine fungus Chaetronium minutum metabolites in 1970 (Figure 1B).5 The stereo structure and the absolute configurations of two cis fused five-membered rings in (+)-chaetocin were elucidated in 1972.6 However, the total synthesis works of (+)-chaetocin were achieved independently by Kim and Movassaghi and Iwasa et al. till 2010 due to its highly comprehensive chemical structure.7-9
The fungal toxin (+)-chaetocin demonstrates strong antineoplastic activities against various cancer cell lines; for example, it exhibits strong HeLa cell inhibition activity with an IC50 of 50 ng/ml.10 Greiner et al. found that (+)-chaetocin had great inhibitory activity against the recombinant HKMT protein SU (VAR) 3–9 (IC50 = 0.6 μM) as well as G9a and DIM5 (IC50 = 2.5 μM and 3 μM, respectively).11 Yuan et al. and Kubicek et al. confirmed that it could widely regulate the enzyme system closely related to epigenetics, such as HKMT, to effectively inhibit breast cancer and pancreatic cancer cells.12, 13 To elucidate its high potency against cancer cell lines, Cherblanc et al. proposed that disulfide bonds were the most crucial factor in blocking the pathways that led to tumor cell death,14-16 which agreed with the SAR research conducted by Iwasa et al. and Teng et al.8, 17
Lung large cell carcinoma and lung adenocarcinoma are the most common types of lung cancer with poor prognosis and account for more than 40% of all lung cancer deaths.18 Although targeted drugs have achieved enormous success in treating non-small-cell lung carcinoma (NSCLC), new mutations of the targets and emerged drug resistance hinder the curative effects.19, 20 Based on these crucial facts, new chemotherapies are always needed to fulfil such circumstances. As specified, (+)-chaetocin demonstrates high anticancer potency and epigenetic regulation merits various cancer cell lines; hence, its anticancer activities towards lung large cell carcinoma and lung adenocarcinoma cell lines were evaluated in this study. The results demonstrated that (+)-chaetocin strongly inhibited lung large cell carcinoma cell lines and effectively induced H460 apoptosis at 10–30 nM concentrations, while cell death occurred directly at 60 nM concentrations due to DNA duplication errors. Cell cycle and transcriptional analysis proved that cell cycle arrest via CDKN1A expression and the comprehensive replicative stress of (+)-chaetocin are key factors in the (+)-chaetocin working mechanisms, as illustrated below.
2 METHODS
2.1 Cell culture
The human NSCLC cell lines H460, H661 and A549 were purchased from the Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences (Shanghai, China). These cell lines were seeded in RMPI-1640 medium (Corning, USA) supplemented with 10% foetal bovine serum (Sigma, USA) and 1% penicillin–streptomycin and cultured at 37°C with 5% CO2. (+)-Chaetocin was purchased from MedChemExpress LLC, Shanghai, China.
2.2 Cell proliferation, apoptosis and cell cycle
The responses to (+)-chaetocin were validated in H460, H661 and A549 cells by measuring the proliferation of these cells. (+)-Chaetocin gradient concentrations between 1.0 and 1.0 uM, or Paclitaxel in gradient concentrations between 0.01 and 10 μM, or vehicle (DMSO) were added for 48 h to these cells, which were seeded into 96-well plates at 2 × 103 cells per well. Cells were treated with 10% CCK-8 solution (GLPBIO, USA) in 90 μl RPMI 1640, incubated for 1 h and then measured at 450 nm using a FlexStation 3 microplate reader (Molecular Devices, USA).
Apoptosis and the cell cycle were detected using an Annexin V-PE/7AAD double staining assay (BD Bioscience, USA) and propidium iodide (PI; BD Bioscience, USA) staining solution. Briefly, cells were seeded at a density of 105 cells/well in a six-well plate and incubated with (+)-chaetocin at different concentrations. After 48 h, the cells were harvested and incubated with Annexin V-PE/7AAD or PI staining solution according to the manufacturer's protocol and analysed using a BD LSRFortessa flow cytometer (BD Bioscience).
2.3 Western blot assay
H460 cells were plated in six-well plates for 48 h and then harvested and lysed in lysis buffer. Proteins were quantified and separated by 10% polyacrylamide gel electrophoresis and then transferred to polyvinylidene fluoride membranes. The membranes with proteins were blocked in 5% nonfat milk and then incubated with primary antibody (Tubulin rabbit monoclonal antibody, Caspase3 rabbit monoclonal antibody, and Bcl2 rabbit monoclonal antibody; BD Bioscience, USA) at 4°C overnight. Horseradish peroxidase-labelled goat anti-rabbit IgG (H+L) was used as the secondary antibody. The immunoreactive bands were visualised with ECL Western Blotting Substrate (Beyotime, China).
2.4 RNASeq analysis
RNA was extracted from washed cells using 500 μl TRIzol reagent (Invitrogen, USA) per well and synthesised into cDNA. After amplification, libraries were prepared for sequencing using the BGISEQ-500 high-throughput sequencing platform (BGI, China). GO bioinformatics analysis and KEGG pathway analysis were used to analyse the sequencing data.
2.5 Statistical analysis
Data are represented as the mean±SEM. Student's t test was used for multiple comparisons. p Values less than 0.05 were considered to be significant.
3 RESULTS
3.1 (+)-Chaetocin inhibited the proliferation of lung large cell carcinoma cell lines
The antineoplastic activity of (+)-chaetocin was investigated using the CCK-8 antiproliferation assay against three different human NSCLC cell lines, including the lung large cell cancer cell lines H460 and H661 and the lung adenocarcinoma cell line A549 at 1.0 μM for the primary tests, while paclitaxel was used as the positive control (Figure 2B). The assay results demonstrated that (+)-chaetocin could efficiently inhibit the proliferation of H460, H661 and A549 cancer cell lines at submicromolar concentrations at 48 h, which was much lower than the working concentration of paclitaxel (Figure 2A).21
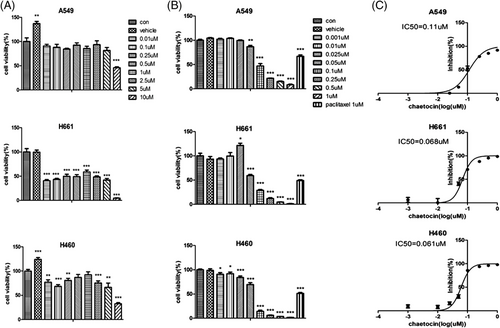
The IC50 values of (+)-chaetocin and the dose–response relationship curves against H460, H661 and A549 cells were evaluated under concentration gradient tests between 1.0 and 1.0 μM at 48 h (Figure 2C). According to these results, (+)-chaetocin efficiently inhibited the proliferation of various lung cancer cell lines tested in a dose-dependent manner, and the IC50 values of (+)-chaetocin against H460, H661 and A549 cells were as low as 61, 68 and 0.11 μM, respectively. This demonstrated that the lung large cell carcinoma cell line would be more sensitive than the lung adenocarcinoma cell line.
3.2 (+)-Chaetocin-induced lung carcinoma H460 apoptosis at low concentrations, while direct cell death occurred at higher concentrations
Based on the excellent antineoplastic activity of (+)-chaetocin against the lung large cell carcinoma cell line H460, (+)-chaetocin-induced cancer cell apoptosis was checked under FACS with an Annexin V-PE/7AAD stain system under 10, 60 and 120 nM concentrations (Figure 3A,B). When H460 cells were treated with 10 nM (+)-chaetocin for 48 h, 8.4% apoptosis was observed, which was similar to the negative control group; when the (+)-chaetocin concentration increased to 60 nM, approximately 3.5% early apoptosis and 44.6% late apoptosis of H460 cells were detected.22, 23 However, when the (+)-chaetocin concentration increased to 120 nM, approximately 8.1% early apoptosis and 60.3% late apoptosis of H460 cells were detected, while many H460 cells had died and broken during 48 h; thus, the total number of detected cells was much lower than that in the other groups.
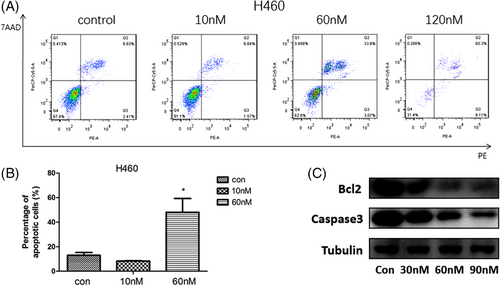
These results may imply that at low concentrations (10-60 nM), (+)-chaetocin effectively injured the lung large cell carcinoma cell line H460, and the cells initiated the apoptosis process after failure of repair. The levels of the antiapoptotic protein caspase 3 and B-cell lymphoma-2 (Bcl2) were also examined in (+)-chaetocin-treated H460 cells by western blot analysis. The levels of caspase 3 and Bcl2 were decreased after drug treatment (Figure 3C). These results suggested that (+)-chaetocin induced apoptosis in human lung large carcinoma cells. However, accompanied with an increased concentration of (+)-chaetocin (60-120 nM), more direct toxic effects were demonstrated against the cells; thus, it was difficult for the carcinoma cells to initiate their apoptosis process as direct cell death was monitored.
3.3 (+)-Chaetocin induced cell cycle arrest in both the G2/M and S phase stages
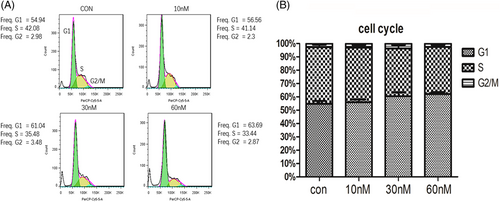
(+)-Chaetocin effectively induced apoptosis in the lung large cell carcinoma cell lines H460 and H661 at low concentrations (< 60 nM) while killing H460 and H661 directly at higher concentrations (60-120 nM). To obtain more information on (+)-chaetocin-treated NSCLC, which implied the possible working mechanism of (+)-chaetocin,24 the cell cycle analysis of the H460 cell line was performed by FACS with PI staining under 10, 30 and 60 nM concentrations (Figure 4A,B). Comparing the 60 nM drug-treated group with the negative control group, the G0/G1 phase cell ratio was increased from 54% to 62%, while the ratio of S phase cells decreased from 42% to 35%. A tendency of an increased G0/G1 phase population and a decreased population in G2/M phase as well as S phase were monitored when H460 cells were treated with increasing concentrations of (+)-chaetocin for 48 h. A decreased ratio of G2/M arrest induced the subsequent apoptosis as monitored above, while a decreased ratio of S-phase cells implied that the duplication of DNA H460 was also hindered with the added drug. Together with the above results, it could be concluded that (+)-chaetocin efficiently inhibited the proliferation of NSCLC cell lines by inducing apoptosis as well as directly toxic effects that hindered DNA duplication, which was consistent with the apoptosis analysis.
3.4 Transcriptional analysis proved the comprehensive replicative stress of (+)-chaetocin in lung large cell carcinoma cell lines
To define the variations in transcriptomic profiles and elucidate the further mechanism of (+)-chaetocin in the lung large cell carcinoma cell line H460, transcriptional research was conducted by RNASeq analysis. Total RNA was extracted from H460 cells and compared between the negative control group and the 60 nM (+)-chaetocin group.
The base excision repair gene clusters and the mismatch repair gene clusters emerged in the top transcriptional disorder gene clusters (p < .05, fold change > 2) after KEGG pathway analysis,25, 26 between the negative control group and the (+)-chaetocin-treated group, demonstrate comprehensive replicative stress of (+)-chaetocin against the H460 cell line (Figure 5A).27, 28
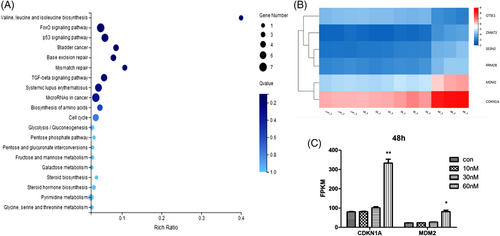
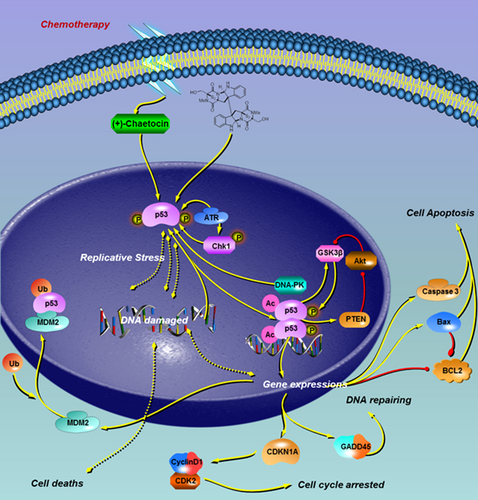
(+)-Chaetocin effectively hindered DNA duplication, and the duplication error increased, while H460 cell death occurred in such situations. Moreover, the cell cycle-tp53 signalling pathway was also greatly affected (Figure 5B), while the transcription of the CDKN1A gene and MDM2 gene were two representative genes affected in (+)-chaetocin-treated H460 cells (Figure 5C).29-31 The results showed that the mRNA level of CDKN1A increased twofold, while the mRNA level of MDM2 also increased twofold when the concentration of (+)-chaetocin increased to 60 nM compared with the control group, which could be reasonable results associated with the damaged DNA. Since CDKN1A expression is always closely controlled by the guardian of the genome protein p53, the corresponding cyclin-dependent kinase inhibitor 1A mediates p53-dependent cell cycle arrest in response to the emerged DNA duplication stress (Figure 6). In response to the DNA duplication errors affected by (+)-chaetocin, p53-induced CDKN1A expression in H460 cells was elevated, since CDKN1A was specifically cleaved by caspase 3, thus leading to a cascade activation of apoptosis following caspase activation.32, 33 In addition, as a negative feedback of activated p53, the expression of the most important suppressor, MDM2, was also elevated for cell survival purposes.34, 35
In addition, the biosynthesis and metabolism of essential factors, including amino acids, sugars and steroid systems, were also greatly affected by (+)-chaetocin, including the valine, leucine and isoleucine biosynthesis gene clusters, the fructose, mannose and galactose metabolism pathway gene clusters and the steroid and pyrimidine metabolic pathways. Taken together, these results demonstrated the comprehensive replicative stress and toxic effects of (+)-chaetocin on large cell carcinoma cell lines.
4 CONCLUSIONS
Lung large cell carcinoma is the most common type of lung cancer with poor prognosis. Although targeted drugs have achieved enormous success in treating NSCLC, new chemotherapy is needed since the emerged drug resistance always hinders curative effects.36 The fungal toxin (+)-chaetocin demonstrated strong antineoplastic activities against the tested lung large cell carcinoma cell lines H460 and H661 at submicromolar concentrations. Further research demonstrated that (+)-chaetocin effectively induced H460 apoptosis at 10–30 nM concentrations, while cell deaths occurred directly at 60 nM concentrations due to DNA duplication errors. Cell cycle and transcriptional analysis proved that cell cycle arrest via CDKN1A expression and the comprehensive replicative stress of (+)-chaetocin are key factors in the (+)-chaetocin working mechanisms, which could be helpful in new chemotherapy drug development.
ACKNOWLEDGEMENTS
The work has been supported by the Natural Science Foundation of Shanghai (20ZR1410400) and the National Natural Science Foundation of China (82173662).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Xin Cao conceived and designed the experiments; Mengjia Qian performed the experiments and analysed the data and Xin Cao and Mengjia Qian have written the manuscript.




