Trans-ethnic polygenic risk scores for body mass index
[Correction added on 06 December 2023; after first online publication missing sub-sections have been added.]
Abstract
Background
Obesity is a complex trait caused by a combination of genetic, environmental and lifestyle factors that contributes to the risks of numerous serious diseases. Predictive measures of body mass index (BMI) hold significant promise, with implications for the prevention and early intervention of obesity, promoting overall improvement in health.
Main body
An effective BMI polygenic risk score (PRS) model can assist with the prediction and early detection of obesity at the individual level, aligning with the objectives of precision medicine in the management of obesity. However, potential health disparities may emerge among under-represented populations with a high prevalence of obesity, primarily due to the lack of genomic data available for these populations. The development of a trans-ethnic (TE) PRS for BMI necessitates collective action from the research community, and requires genomic data from diverse populations.
Conclusion
The current BMI-PRS model exhibits moderate performance, which could be improved in several key areas: integrating genomic information through TE GWAS studies, including admixed populations, considering gene–environment interactions, implementing advanced machine learning techniques, and incorporating genomic-informed risk assessment (GIRA) with the inclusion of family history, environmental and lifestyle factors for the risk prediction.
1 BODY MASS INDEX AND ITS SIGNIFICANCE FOR HEALTH
Body mass index (BMI) is the traditional numerical measurement, calculated by (bodyweight/height2), that enables the detection of over-weight and diagnosis of obesity. Obesity is a complex trait caused by a combination of genetic, environmental and lifestyle factors.1 The prevalence of obesity varies significantly among different populations.2 High prevalence of obesity is seen in Latin Americans,3 Middle East populations4 and Pacific Islanders.5 In the United States, Hispanic and African Americans have higher rate of obesity than European Americans.6 Understanding these disparities is crucial in developing targeted interventions and strategies to address the specific needs of these populations.
Obesity causes adipose tissue dysfunction and leads to chronic low-grade inflammation, which serves as a key contributor to the development of chronic diseases associated with obesity.7 Excessive bodyweight also places mechanical stress on heart, contributes to respiratory difficulties and paroxysmal nocturnal dyspnoea (PND), and impacts joint health. As a result, obesity contributes to the risk of a number of serious diseases, including cardiovascular diseases (e.g., hypertension, heart failure and coronary artery disease),8 chronic metabolic diseases (e.g., metabolic syndrome, type 2 diabetes and non-alcoholic fatty liver disease [NAFLD]) and respiratory diseases (e.g., asthma and chronic obstructive pulmonary disease [COPD]).9, 10 Moreover, obesity is a known risk factor for several types of cancer.11 The chronic inflammation and observed hormonal imbalance associated with obesity can promote the development of certain cancers, including breast, colon, ovarian and pancreatic cancers.12 Beyond its physical implications, obesity also has a substantial impact on mental health.13 The psychological consequences of obesity, such as body image dissatisfaction, low self-esteem and social stigmatization, further contributes to the negative burden experienced by individuals with obesity.14
2 BMI AND ITS RISK FACTORS
The awareness of BMI and its implications in reference to health extends beyond a simple numerical measurement. Genetic variations have been found to play a significant role in the susceptibility to obesity by influencing various physiological processes such as metabolism, fat storage and distribution, and appetite regulation. The fat mass and obesity-associated gene (FTO) is the first gene identified of association with the genetic susceptibility of obesity.15 Tews et al. showed that FTO-deficient adipocytes upregulate uncoupling protein 1 (UCP1) expression, resulting in enhanced mitochondrial uncoupling and energy expenditure.16 Haupt et al. observed significant impact of FTO variation on whole-body fat distribution.17 Both common18 and rare19, 20 variants of the melanocortin 4 receptor gene (MC4R) have been associated with obesity. MC4R is expressed mainly in hypothalamus, and is the central melanocortin receptor regulating appetite and energy expenditure.21, 22
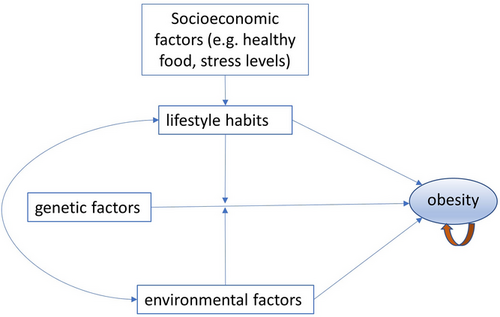
Genetic factors alone do not account for the entire obesity epidemic. Long-term environmental factors and lifestyle habits also play a significant role. Dietary choices (high sugar, high fat, low fibre) and lack of physical activity have been strongly associated with the development of obesity23 (Figure 1). It is important to recognize that the prevention of obesity through healthy dietary habits and regular exercise is easier and more effective than treating it once it has been diagnosed. By focusing on prevention and early intervention, individuals can reduce the risk of obesity-related health complications. Furthermore, obesity itself can create a vicious cycle that compounds its presence.24 The mechanical stress and joint injuries resulting from excess weight can limit physical exercise of an obese patient, further exacerbating the problem. This cycle emphasizes the importance of early intervention and prevention efforts to break the cycle and promote better health outcomes. For these reasons, the prediction of BMI has important implications in the prevention/early intervention of obesity and overall health promotion.
3 POLYGENIC RISK SCORE (PRS) FOR BMI
Polygenic risk scores (PRS), which combines information from multiple genetic variants across the genome, have emerged as a promising tool for assessing the genetic contribution to complex traits such as BMI. Successful PRS models have been developed for a number of common diseases, for example, breast cancer,29 type 2 diabetes30 and coronary artery disease.31 For type 1 diabetes (T1D), the area under the ROC curve (AUC) for the prediction of the disease is up to 0.900.32, 33 Common genetic variation accounts for over 20% of variation in BMI.34 A large number of BMI-associated common variants have been identified by genome-wide association studies, for example, the GWAS meta-analysis of BMI in European population by the GIANT consortium.35 The BMI-associated genetic markers identified in the previous GWASs enable the development of BMI-PRS. In contrast, including rare variants associated with BMI has no obvious improvement of PRS models built using common variants.36
-
An effective BMI-PRS model may assist the prediction and early detection of obesity in a person. Early identification of individuals at increased genetic risk for obesity may enable the implementation of targeted preventive measures and interventions, aligning with the goals of precision medicine. With this information, healthcare professionals can develop personalized strategies focused on modifying environmental and lifestyle factors, effectively reducing the risk of obesity development. Dashti et al. demonstrated a correlation between obesity PRS and objectively measured food purchases at the workplace, including the quality, quantity and timing. These findings indicate the potential for personalized workplace wellness programmes to target individuals with a high risk of obesity.37 As other examples, the genetic stratification of obese patients with pro-opiomelanocortin (POMC), leptin receptor (LEPR) or melanocortin-4 receptor (MC4R) deficiency has resulted in the development of a groundbreaking obesity treatment using the MC4R agonist (setmelanotide).38, 39
-
A PRS model can aid in assessing the overall impact of genetic factors relative to environmental and lifestyle factors on an individual's obesity risk. This enables a more precise understanding of the different factors to an individual's risk. Healthcare professionals can tailor interventions to address the specific needs of each patient. Individuals with a high genetic risk for obesity may require more intensive interventions, such as specialized dietary plans or increased physical activity, to counterbalance their genetic predisposition.
-
At the population level, a PRS for BMI may aid in risk stratification of patients and resource allocation. By identifying individuals at high genetic risk for obesity, healthcare systems can allocate resources more efficiently, focusing on interventions and support for those who need them most. This targeted approach ensures that high-risk individuals receive the necessary attention and resources to prevent obesity-related complications. Furthermore, by leveraging population-level PRS data, public health initiatives can be tailored to specific communities or demographics with higher genetic predisposition, leading to more effective preventive strategies and reduced healthcare burden.
-
A PRS model for BMI may also have implications in research and drug development by facilitating the identification of genetically susceptible subgroups, and appropriate stratification of research subjects. The implications of a BMI-PRS model extend beyond clinical practice and public health. In the field of research and drug development, the ability to identify genetically susceptible subgroups through PRS analysis can revolutionize study design and participant stratification. In a case–control study, the PRS may enable better match of cases and controls. In particular, researchers can recruit individuals with specific PRS profiles to develop targeted interventions. This approach not only enhances the scientific validity of studies but also improves the likelihood of successful outcomes by tailoring interventions to genetically susceptible subgroups. Analogously, Damask et al. showed that individuals with a high PRS for coronary artery disease (CAD) experienced more significant absolute and relative risk reduction when treated with the PCSK9 blocking antibody alirocumab.40 In this case, the high PRS serves as an independent tool for patient stratification.
Importantly, a previous report highlights the association of BMI-derived PRS across the lifespan.41 Currently, the performance of PRS for BMI prediction is relatively modest. Our recent PRS model for extreme BMI, that is, the top 1% and 5% has a moderate AUC of 0.703–0.736 with an acceptable predictive power in two large European populations, the Nurses’ Health Study (NHS) and Nurses’ Health Study II (NHSII).42 As further research and advancements continue, the BMI-PRS model is expected to evolve and enhance its predictive power, ultimately contributing to more effective obesity prevention and treatment strategies.
4 POTENTIAL HEALTH DISPARITIES
The PRS for BMI was facilitated by the comprehensive identification of SNPs associated with BMI by the previous large-scale GWAS by the GIANT consortium.34 The GWAS meta-analysis by the GIANT consortium, including 339 224 individuals of European ancestry,34 was the basis of our PRS model.42 The updated GWAS by the GIANT consortium and the UK Biobank included approximately 700 000 individuals of European ancestry.35 The GWAS by Heilbron et al. included 2 433 011 European.43 In contrast, GWASs on populations other than European are heavily under-represented (Table 1). The large sample GWAS by Huang et al. included 509 429 Europeans with 16 962 individuals of other ethnicities, including African American or Afro-Caribbean, East Asian, Hispanic or Latin American, and South Asian.44 Notably, the exome study by Akbari et al. included 549 780 European and 95 846 Hispanic or Latin American.45 However, as an exome study cannot capture variants in non-coding regions, its contribution to PRS is modest.
| Study | Sample number and ancestry | Genotyping |
|---|---|---|
| Locke AE34 | 339 224 individuals of European ancestry | GWAS arrays |
| Yengo L35 | ∼700 000 individuals of European ancestry | GWAS arrays |
| Heilbron K43 | 2 433 011 European | GWAS arrays |
| Huang LO44 | 509 429 Europeans and 16 962 individuals of other ethnicities | GWAS arrays |
| Akbari P45 | 549 780 European and 95 846 Hispanic or Latin American | Exome sequencing |
With the high prevalence of obesity in under-represented diversity populations, the discrepancy of lack of genomic data in populations other than European raises potential inequalities in the application of PRS models developed solely based on European data. A PRS model created by European data may not be applied to other populations. The marker SNPs in a European PRS may have different linkage disequilibrium (LD) in other populations. Two variants in LD are inherited together more often than expected by chance, while the LD may vary by populations related to founder effects, population bottlenecks, natural selection, genetic drift and admixture.46 Due to LD, two causal SNPs in BMI do not have completely independent effects.
Furthermore, environmental factors and lifestyle habits may have varied interactions with genetic factors across populations (Figure 1). Corella et al. showed that a high intake of saturated fatty acids strengthens the association between FTO variants and BMI.25 Conversely, physical activity attenuates association between FTO variants and BMI.26 Children carrying the protective FTO genotype may be more protected by a favourable social environment.27 Given the significant difference of environmental factors and lifestyle habits across human populations, a European PRS may not perform adequately in other human populations. Consequently, a PRS for BMI only available for European may lead to healthcare disparities, resulting in less effective prevention and intervention of obesity in under-represented populations. To address this issue and promote equitable healthcare, there is a pressing need for the development of a trans-ethnic (TE) PRS for BMI and obesity, requiring proactive action from the research community to ensure diverse representation and the collection of genomic data from different populations. A concerted effort to acquire and incorporate genomic information from under-represented populations in the development of PRS models is essential to overcome potential health disparities.
5 GLOBAL GENOMIC DATA SHARING
The global sharing of genomic data is a crucial aspect of developing and testing a TE PRS, which requires genomic data of diverse populations. However, there are numerous challenges in facilitating the international sharing of genomic data, encompassing both ethical and legal considerations. Consent agreements from participants in genomic studies may not explicitly encompass global data sharing. Consent agreements are typically obtained within the context of a specific research project and may not explicitly cover the broad sharing of genomic data with researchers from different countries and institutions. To address this issue, efforts are needed to enhance consent processes and develop standardized frameworks that encompass global data sharing while upholding ethical principles and respecting participant autonomy. Moreover, legal and regulatory frameworks differ across countries. Certain countries have regulations that prohibit the cross-border transfer of genomic data. These regulations aim to safeguard privacy and protect sensitive information. However, they can hinder international collaborations and impede the progress of genomic research. Overcoming these barriers necessitates international collaborations aimed at addressing these challenges and facilitating responsible and secure data sharing.
To tackle these challenges and promote global genomic data sharing, various initiatives and organizations have emerged. The Global Alliance for Genomics and Health (GA4GH)47 and the Global Genomic Medicine Collaborative (G2MC)48 are leading efforts to develop frameworks and standards for responsible data sharing across international borders. These initiatives aim to establish guidelines, tools and best practices that enable secure and ethical sharing of genomic and clinical data. By fostering collaboration and building consensus among researchers, policymakers and stakeholders, these organizations facilitate the sharing of genomic data while addressing ethical, legal and technical considerations. The International HundredK+ Cohorts Consortium (IHCC, https://ihccglobal.org/) was established in 2019 as a collaborative effort involving GA4GH and G2MC. The IHCC aims to accelerate genomic research by leveraging large-scale genomic and phenotypic data from diverse population cohorts worldwide, comprising 103 cohorts in 43 countries involving nearly 50 million participants.49
For our IHCC study on a TE BMI-PRS, the Center for Applied Genomics (CAG) at the Children's Hospital of Philadelphia (CHOP) with one of the world's largest paediatric biobank50 created the PRS model. In 57 613 randomly selected CAG samples from different populations, AUC = 0.735 was acquired with the fraction 0.1 of causal markers to predict top 1% BMI. Without the need for data transfer and time-consuming data sharing agreements, the detailed protocols and all required files to generate the PRS were shared with the participating cohorts. The participating IHCC cohorts in this study included the Nurses’ Health Study I (over 17 000 participants of European ancestry), the Nurses’ Health Study II (over 12 000 participants of European ancestry), the Shanghai Women's Health Study (SWHS, ∼75 000 Chinese women),51 the Shanghai Men's Health Study (SMHS, 61 480 Chinese men),52 the Norwegian Mother, Father and Child Cohort Study (MoBA, 50 290 Northern European adult males and females analyzed in this study), the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil, 9333 civil servants in Brazil analyzed in this study)53, 54 and the INTERVAL BioResource (38 319 Europeans included in this study).55 As the lead group, we shared the protocol of the PRS models, as well as the SNPs and weights, with the IHCC collaborators for assessment by the international sites. Harmonization of SNP alleles in the PRS model was by comparing with the reference alleles of the TOPMed imputation.
6 TE PRS FOR BMI
While population-specific PRS models tend to have optimal performance, a TE PRS has the advantage of broader applicability, particularly in populations with limited genomic data. Ideally, a TE PRS needs to incorporate disease-associated genetic variants identified from diverse populations.56 In the case of the TE PRS for BMI, with limited data available from TE GWAS, we made efforts on developing a TE PRS based on the GWAS results in Europeans, while accounting for population-specific linkage disequilibrium (LD) patterns using a Bayesian approach as implemented in LDpred.57 Compared to the PRS approaches of LD pruning followed by p-value thresholding (P+T), LDpred demonstrated its improved performance by taking into account of LD information between genetic markers,57 thereby retaining genetic information that might be lost through LD pruning. For example, two genetic markers in LD each may have causal effect. While different populations have different genetic risk of obesity, the underlying assumption of our approach is that causal SNPs of BMI in proximity tends to be in LD in different populations.
-
Positive selection: Positive selection, also known as adaptive selection, is a process in evolution by which beneficial genetic variants increase in frequency within a population over time.58 Throughout human history, causal variants associated with obesity may have been subjected to positive selection if the variants promoting energy storage and fat deposition had provided advantages against food scarcity in the past.59 However, in modern environments characterized by abundant food availability, these advantageous variants may no longer confer the same benefits. As an evolutionary genetic signature, LD might develop between the causal variants under positive selection and nearby variants through selective sweep or genetic hitchhiking.60
-
Negative selection: Negative selection, also known as purifying selection, refers to the process by which deleterious or harmful genetic variants are removed from a population over time.58 It acts to reduce the frequency of alleles that have negative effects on an organism's fitness, survival or reproductive success. Significant purifying selection has also been suggested in obesity genes in human, for example, MC4R related to eating behaviour.61 Variants in the MC4R gene have been associated with increased susceptibility to obesity and disturbances in eating behaviour.62 Individuals with such mutations might have faced challenges in finding sufficient food resources or might have had impaired metabolism, negatively impacting their reproductive success and overall survival. Purifying selection can lead to extended LD through background selection, selective constraint or genetic hitchhiking.63, 64
-
Balancing selection: Balancing selection refers to a type of natural selection that maintains genetic variation within a population by favouring multiple alleles of a gene rather than favouring a single allele.65 One possible mechanism of balancing selection is when different alleles confer advantages in different environments or under different selective pressures, leading to a balanced distribution of genetic diversity. FTO is an interesting example of balancing selection in the context of obesity. FTO plays a role in energy homeostasis and has been implicated in the regulation of bodyweight and fat mass.66 It is upregulated during food deprivation, suggesting its involvement in the response to fasting and energy balance.67 Additionally, FTO is expressed in neurons of feeding-related nuclei of the brain, supporting its role in appetite regulation.67 Balancing selection signature has been detected at the FTO locus in human populations, with different alleles at this gene being maintained at relatively high frequencies.68 The FTO locus might have experienced a dynamic evolutionary history, potentially due to the selective advantages conferred by different alleles in different environmental contexts. For instance, one allele may be beneficial in terms of energy conservation during periods of limited food availability, while another allele may be advantageous for efficient energy utilization when food is abundant. Different balancing selection among human populations is related to environmental factors, and may have varied effects on LD in different populations.
Besides natural selection, other evolutionary forces, such as genetic drift, migration patterns, admixture events, recombination rates and assortative mating, can also contribute to population-specific LD patterns. Genetic drift can be a dominant force shaping LD patterns in small isolated populations.69 Conversely, migration, or gene flow, facilitates the movement of genetic material between populations, thereby reducing population-specific LD patterns.70 Admixture events can introduce new alleles into a population or dilute existing LD patterns.71 Recombination breaks down LD, while lower recombination rates can maintain LD over longer stretches of DNA.72 Assortative mating, which involves individuals preferring mates with similar characteristics, leads to increased homozygosity and the preservation of LD.73
Due to significant differences of environmental factors and evolutionary forces across populations, LD patterns of BMI-causal SNPs may vary. Shown in our study, our TE PRS models were based on the meta-LD matrices, including four major human populations, that is, African American (AA), Hispanic American (HAMR), East Asian (ASN) and Northern European (EUR). Our data showed that the TE PRS outperformed the ancestry-specific models in the Chinese and Brazilian populations, whereas there is no appreciable loss in predictive power in European ancestry individuals when using a TE score. However, as shown in our study, the TE PRS for BMI has a poor performance in the admixed Brazilian population, although it outperforms the European PRS.42 Increased genetic diversity and unique genetic drift and selection pressures contribute different LD patterns. In particular, genetic admixture generates LD blocks inherited from each ancestral population. Over time, as recombination events occur in subsequent generations, LD tends to decrease. The meta-LD matrices of non-admixed ancestral populations may not be able to account for the LDs properly in the admixed Brazilian population. It is worth mentioning that in addition to LD, population-specific mutations may also contribute to the limited portability of a TE PRS.74 This highlights the importance of increasing diversity in genetic studies by including under-represented populations.
7 PERSPECTIVE
-
Multi-ethnic genetic data: One potential avenue for enhancing TE PRS performance is through dedicated TE GWAS studies. Duncan et al. demonstrated that European-derived PRSs had lower predictive performance in non-European ancestry samples and emphasized the importance of improving methodological approaches to account for linkage disequilibrium and variant frequencies when applying polygenic scoring in diverse populations.75 With TE GWAS statistics, a PRS tool, for example, PRS-CSx incorporating continuous shrinkage, may improve the accuracy and robustness of PRS by integrating GWAS summary statistics from multiple populations.76 By including diverse ancestral populations, particularly those representing the admixed populations of interest, researchers can capture the genetic markers and LD patterns specific to these populations, leading to more accurate prediction models for BMI in admixed populations.
-
Gene–environment interaction analyses: Notably, the study by Graff et al. underscored the significance of considering genome-wide physical activity interactions, as they offer a powerful means to unearth novel genetic factors involved in obesity.77 Considering the independent and interactive effects of genetic factors, environmental factors and lifestyle habits on obesity, large-scale gene–environment interaction analyses are warranted, particularly in under-represented population groups. For instance, significant efforts have been devoted to studying post-menopausal African American and Hispanic women.78
-
Admixed population analysis techniques: Conducting large-scale GWAS in admixed populations, such as the Brazilian population, presents numerous unique challenges. The high genetic diversity resulting from the mixing of ancestral populations can complicate the identification of disease-associated variants and the estimation of their effects. Additionally, the complex population structure, encompassing multiple ancestral contributions, requires sophisticated analytical approaches to disentangle the underlying genetic architecture. Notably, Liu et al. developed a novel identity-by-state (IBS) approach for genotype imputation in admixed populations and achieved consistently higher imputation quality.79 Ancestry weighting assigns different weights to genetic variants, and improves the accuracy of GWAS in admixed populations by considering the differences in allele frequencies across ancestries.80
-
Machine learning approaches: The effectiveness of machine learning data modelling in predicting complex diseases stems from its ability to effectively process interactive, multi-dimensional data.81 Machine learning algorithms have shown promise in improving the accuracy of TE PRS.82 Machine learning methods can handle large-scale genetic data and identify complex patterns that traditional statistical approaches might miss.
The challenges and opportunities require extensive collaborations and innovative mechanisms for data sharing among international research consortia, such as the IHCC, to pool resources and expertise. Beyond genetic factors, taking into account other risk factors of BMI may enable more accurate prediction of BMI. For example, Welten et al.83 took into account parental BMIs, birthweight, sex and a number of socioeconomic and environmental factors, and acquired AUC = 0.845 without PRS to predict sex/age-specific overweight in the Netherland population. Family history is a valuable and cost-effective tool that should not be neglected in disease risk prediction. It is based on observable patterns of diseases among relatives, and relies on the accuracy and completeness of the information provided by individuals. However, there may be cases where family history is not evident. In such situations, genetic testing may be necessary to complement the information obtained from family history. Genomic informed risk assessment (GIRA)84 including major environmental and lifestyle factors in addition to PRS may have improved accuracy in the prediction of BMI.
AUTHOR CONTRIBUTIONS
Conceptualization, H.H. and H-Q.Q.; writing—original draft preparation, H-Q.Q.; writing—review and editing, H-Q.Q., J.J.C., and H.H.; supervision, H.H. All authors have read and agreed to the submitted version of the manuscript.
ACKNOWLEDGEMENTS
None.
CONFLICT OF INTEREST STATEMENT
The authors declare no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
FUNDING INFORMATION
International HundredK+ Cohorts Consortium (IHCC); Institutional Development Funds from Children's Hospital of Philadelphia to the Center for Applied Genomics; Children's Hospital of Philadelphia Endowed Chair in Genomic Research to HH.
ETHICS APPROVAL
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
Not applicable.




