Lymph node metastasis in oral squamous cell carcinoma: Where we are and where we are going
[Correction added on 06 December 2023; after first online publication missing sub-sections have been added.]
Abstract
Background
Oral cancer is a common malignant tumour in the head and neck region, with over 90% of cases being oral squamous cell carcinoma (OSCC). Lymph node metastasis (LNM) is a significant adverse prognostic factor in OSCC; however, the mechanism of LNM in OSCC remains unclear, while the strategies for managing cervical lymph nodes (CLNs) in OSCC continue to evolve. At present, neck dissection is necessary for the vast majority of OSCC patients, but complications of surgery can significantly impair patients’ postoperative quality of life. A recent clinical trial discovery indicates that immunotherapy can activate anti-tumour T cells in nearby lymph nodes, leading to vibrant discussions among clinicians about the importance of preserving lymph nodes during surgical treatment.
Aim
This review aims to provide an overview of where we are: the current knowledge of OSCC LNM patterns and management strategies, and where we are going: prospects for clinical and basic research on the issue of OSCC LNM in the era of cancer immunotherapy.
Result
We summarize the general patterns and management strategies of OSCC LNM. The process of LNM in OSCC encompasses four stages: Preparation, Unleash, Migration, and Planting (‘PUMP’ principle). We propose adopting the ‘PRECISE’ model, an overall workflow that includes seven elements—prevention, radiology, preoperative evaluation, chemotherapy, immunotherapy, surgery, and postoperative evaluation—for neck management in OSCC, promoting personalized precision medicine augmented by neoadjuvant therapy. We further discussed recent advances in clinical and basic research on OSCC LNM and provided an outlook on future research directions.
Conclusion
Over the past two centuries, our understanding and management strategies of LNM in OSCC have evolved, seeking more precise and personalized approaches to reduce patient burden and enhance survival and quality of life. Further research should explore lymph nodes’ role in cancer immunotherapy and OSCC interactions, leading to better, less invasive treatments and improved outcomes.
1 INTRODUCTION
In 2020, globally, there were approximately 377 713 new cases and an estimated 177 757 deaths from cancers of the lip and oral cavity. Collectively, these cancers rank as the 18th most common neoplasm worldwide.1 Over 90% of these cancers are squamous cell carcinomas.2 Lymph node metastasis (LNM) is the most adverse prognostic factor of oral squamous cell carcinoma (OSCC), with an incidence rate of approximately 40%.3, 4 According to the eighth edition of American Joint Committee on Cancer (AJCC) staging manual, patients without LNM have an overall 5-year survival rate exceeding 80%, whereas those in the N3 stage have a significantly lower 5-year survival rate of approximately 20%.5 Currently, the mechanism of LNM in OSCC remains unclear, while the strategies for managing cervical lymph nodes (CLNs) in OSCC continue to evolve.
At present, neck dissection is necessary for the vast majority of OSCC patients. However, complications such as nerve damage, hematoma, vascular complications, hypoparathyroidism, chyle fistula, pneumothorax, and wound complications from surgical trauma can significantly impair patients’ postoperative quality of life.6 A recent clinical trial discovery indicates that immunotherapy can activate anti-tumour T cells in nearby lymph nodes,7 leading to vibrant discussions among clinicians about the importance of preserving lymph nodes during surgical treatment.
This review provides an overview of where we are: the current knowledge of OSCC LNM patterns and management strategies, and where we are going: prospects for clinical and basic research on the issue of OSCC LNM in the era of cancer immunotherapy. However, palliative treatment for unresectable advanced cancer is currently beyond our scope. The definition of oral cancer, from an epidemiological perspective, can be both confusing and complex.8 To avoid ambiguity, ‘oral cancer’ in this review refers to squamous cell carcinomas of the oral cavity (OSCC) originating from the mucosal lip, anterior tongue, buccal mucosa, floor of mouth, hard palate, alveolar ridge, and the retromolar trigone, following the National Comprehensive Cancer Network (NCCN) guidelines,9 and may be expanded appropriately in accordance with the original context of the referenced literature.
2 TREATMENT HISTORY OF LNM IN OSCC
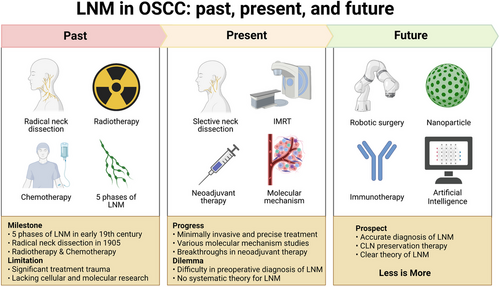
The significance of nodal metastases was first documented in medical literature around 1790. In the early 19th century, researchers defined five distinct phases of lymphatic spread: liberation, transportation, deposition, establishment, and growth of malignant cells.10 This theory closely aligns with our current understanding of the lymph node metastasis process, barring the stage of premetastatic niche changes before metastasis. The history of neck lymph node dissection dates back to 1837 when Warren reported an improved method for neck tumour removal.11 In 1905−1906, Crile pioneered the systematic en bloc dissection of cervical lymphatic system. This surgical technique, widely known as radical neck dissection (RND), emerged as the gold standard operation for neck metastasis in subsequent decades.12 However, due to constraints in medical technology such as antibiotics and blood transfusions, and the surgical complexity, radiotherapy emerged as the preferred treatment for OSCC and its LNM between the two World Wars.13
In 1950s, the small field irradiation technique for treating cervical LNM of squamous cell carcinoma was introduced.14 By 1963, Osvaldo Suarez published the initial and methodical technique for performing a functional neck dissection (FND), laying the groundwork for subsequent selective neck dissection (SND).15, 16 Concurrently, chemotherapy was proposed as an ‘adjuvant’ treatment to neutralize tumour cells already present in the lymph nodes.17 In 1990, research by Shah et al. on the distribution of regional metastasis laid the foundation for investigating the feasibility of selective neck dissection in OSCC.18 In the pioneering work in 1999, Shoaib et al. successfully laid the groundwork for sentinel lymph node biopsy (SLNB) in the cervical region, harnessing the potential of 99 m-Tc for the precise identification of sentinel lymph nodes.19 Fast forward to February 2023, a landmark study conducted by Rahim et al. shed light on the previously unexplored crucial function of human cervical lymph nodes in tumour immunotherapy.7 Their groundbreaking findings served as an initial revelation of this vital role within the scientific community. Figure 1 highlights the key milestones in the research of LNM in OSCC.
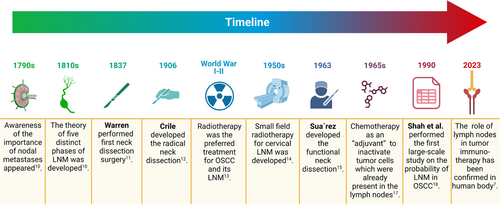
3 GENERAL PATTERNS OF LNM IN OSCC
3.1 Molecular patterns of LNM
Over the past half century, there have been significant advancements in the study of cellular and molecular mechanisms of LNM. We now understand LNM as a continuous, intricate, and dynamic process. Adopting a holistic approach to this issue can provide deeper insights into the molecular patterns of LNM. Recent literature reviews have given a comprehensive summary of the current physiological and pathological research on LNM, with a focus on the cellular and molecular levels.20, 21 This article will specifically spotlight the molecular mechanisms of LNM from a holistic and dynamic perspective. Generally, the process of LNM can be summarized into four stages: Preparation, Unleash, Migration, and Planting, collectively referred to as the ‘PUMP’ principle by us (Figure 2).
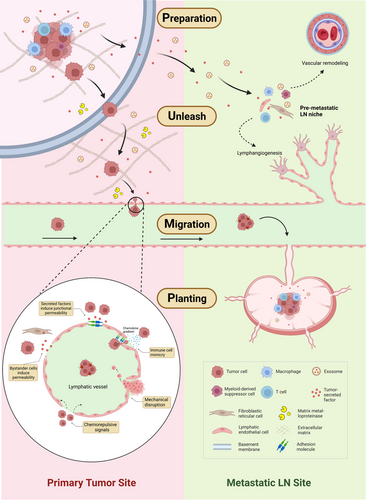
The Preparation stage of LNM occurs prior to metastatic spread when the primary tumour undergoes changes in readiness for the remodeling of the draining lymph node, a process also known as premetastatic niche formation. This stage involves the secretion of tumour-derived factors and extracellular vesicles (EVs) that are subsequently transported by lymphatic vessels. These biological molecules recruit macrophages, myeloid-derived suppressor cells (MDSCs), and regulatory T cells, creating an immunosuppressive microenvironment. Concurrently, the proliferation of lymphatic endothelial cells (LECs) and fibroblastic reticular cells (FRCs) is induced by tumour-derived factors and EVs. These cells regulate the secretion of chemokines and other related factors in the lymph nodes, leading to vascular remodeling and lymphangiogenesis at both the primary tumour site and pre-metastatic site.22
The Unleash phase ensues as various biological factors, such as hepatocyte growth factors, reduce cell adhesion, leading to the detachment of cancer cells from the primary tumour and infiltration into the extracellular matrix (ECM).23 The tumour then secretes matrix-degrading enzymes like matrix metalloproteinases (MMPs), which degrade the ECM, creating a pathway for tumour cell migration and promoting the invasion of cancer cells into lymphatic vessels.24
The Migration stage of LNM involves cancer cells moving towards the lymphatic vessels, guided by the chemotactic gradient of chemokines within the tissue. Upon adherence to the lymphatic endothelium, they breach the endothelial cell barrier and enter the lymphatic lumen. Physical compression caused by tumour growth, or chemorepulsive signals released by LECs in response to tumour cells, can directly lead to the perforation of the lymphatic endothelium. Tumour-secreted factors and bystander cells can augment the permeability of lymphatic endothelium. Tumour cells may also enter lymphatic vessels by mimicking immune cells.21 Within the lymphatic vessels, which contain intraluminal valves to ensure unidirectional lymph flow, the cancer cells are transported through the lymphatic stream and ultimately drain into sentinel lymph nodes. The pumping force for lymph flow is generated by active contractions of specialized lymphatic muscle cells and various passive factors such as pulsatile blood flow, skeletal or smooth muscle contraction, fluid pressure gradients, and gravity.20
In the final Planting stage, cancer cells establish and proliferate in the lymph node, leading to the occurrence of LNM. The cancer cell phenotype undergoes continuous changes throughout the whole four stages.21, 22, 25 The molecular mechanisms underlying tumour LNM are not yet fully understood, given that they involve a complex, multifactorial process in molecular biology characterized by multiple steps and ongoing regulation. While previous studies have often focused on specific stages of LNM, future research should direct more attention towards changes in relevant molecules and cells throughout the entire LNM cycle.
3.2 Anatomical patterns of LNM
The current, most widely accepted method for classifying CLNs is the six-level classification system recommended by the American Head and Neck Society.26 This classification categorizes CLNs into level I-VI. OSCC primarily involves level I-V. Notably, the primary lymphatic drainage groups of the lips correspond to levels IA and IB. The lymphatic drainage areas for the anterior two-thirds of the tongue are level IB, II, and III, while the anterior third of the tongue can drain into level IA. The floor of the mouth primarily drains into level IB, II, and III, with the anterior portion draining into level IA. The retromolar triangle mainly drains into level IB, II and III.27
However, this classification system, guided by clinical and imaging considerations, excludes certain lymph nodes such as parotid, facial, and lingual nodes from the level system. A recent study detailed the lymphatic drainage system through gross anatomical research and subsequently developed a classification system for CLNs based on RND. This study suggested that draining lymph nodes of OSCC include submandibular nodes, facial nodes, submental nodes, superior deep cervical nodes, superficial parotid nodes, and jugulodigastric nodes.28 The lymphatic drainage from different sites in the oral cavity is summarized in Table 1.
| Primary site | Draining lymph nodes |
|---|---|
| Upper lip and lateral part of the lower lip | Submandibular and facial nodes |
| Center of the lower lip | Submental nodes |
| Gingiva and anterior part of the hard palate | submental, submandibular, and facial nodes |
| Posterior part of the hard palate | Superior deep cervical and superficial parotid nodes |
| Anterior part of the oral floor | Superior deep cervical nodes or pass through submental nodes |
| Buccal mucosa | Submandibular |
| Tongue, marginal | Submental and submandibular, superior deep cervical nodes, jugulodigastric nodes |
| Tongue, dorsal | Submental and submandibular, jugulodigastric nodes |
| Tongue, basal | Superior deep cervical nodes |
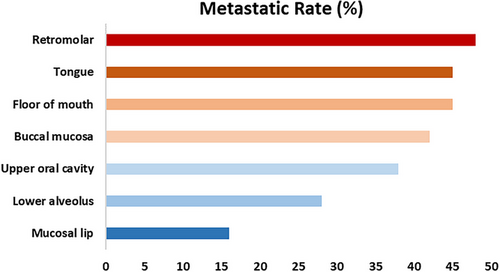
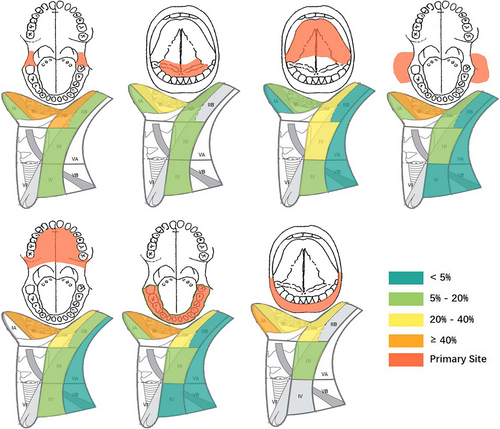
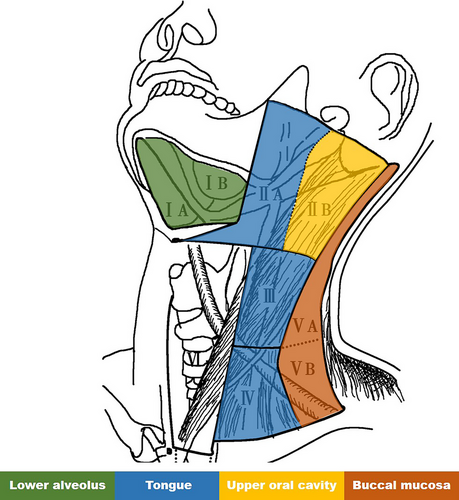
According to recent large-scale studies, the rates of LNM in OSCC across different primary sites are as follows: buccal mucosa-42%; lower alveolus-28%; retromolar-48%; floor of mouth-45%; tongue-45%; mucosal lip-16%; upper oral cavity-38% (Figure 3).29-32 As previously mentioned, lymphatic drainage from OSCC typically first reaches levels I and II lymph nodes before progressing to level III. The ‘cascade theory’33 indicate that OSCC LNM should first appears in proximate levels I, II and III lymph nodes, with fewer metastases in level IV and V. A recent large-scale study indicate that 91% of LNM in OSCC occur in levels I, II and III, while metastasis rates in levels IV and V are relatively low at 4.7% and 3.3%, respectively.34 However, the limitation of this study is the small sample size for cancers originating from the upper oral cavity and lips, potentially leading to a significant bias. The patterns of LNM from different subsites within the oral cavity are detailed in Table 2 and Figure 4, with Figure 5 highlighting primary sites with the highest rates of LNM in each level. Due to limited data on the lip, retromolar, and floor of the mouth were excluded.
| Primary Site | Case number | IA | IB | IIA | IIB | III | IV | V |
|---|---|---|---|---|---|---|---|---|
| Tongue | 178 | 4.8% | 19.9% | 35.1% | 2.7% | 22.1% | 7% | 3.8% |
| Buccal mucosa | 151 | 6.5% | 40.4% | 14.6% | 3.4% | 7.3% | 3.7% | 4.9% |
| Lower alveolus | 86 | 18.4% | 46.9% | 24% | 3.1% | 11.5% | 4.8% | 4.9% |
| Floor of mouth | 15 | 14.3% | 36.4% | 18.2% | 0% | 18.2% | 9.1% | 0% |
| Lip | 9 | 40% | 33.3% | 33.3% | 0% | 16.7% | 0% | 0% |
| Retromolar trigone | 16 | 6.3% | 58.8% | 41.2% | 6.3% | 5.9% | 7.1% | 0% |
| Upper oral cavity* | 66 | 0% | 43.2% | 27 | 8.1% | 13.5% | 5.4% | 2.7% |
- * Upper oral cavity data were extracted from Doll et al.29.
In early stages, OSCC can develop occult LNM, characterized by lymph nodes that appear negative during preoperative assessments, such as palpation or imaging, but are found to contain cancer cells upon pathological examination after neck dissection. About 20%−40% of early-stage OSCC patients exhibit occult cervical LNM.35-38 Additionally, LNM correlates with the prevalence of distant cancer metastasis, with rates of 5.1%, 11.3%, 19.1%, and 28.9% reported in N0, N1, N2 and N3 stages, respectively (p < .001).39
4 NECK MANAGEMENT FOR OSCC
4.1 Preoperative assessment of CLNs
The primary clinical methods employed for CLNs assessment include palpation, ultrasonography (US), computed tomography (CT), magnetic resonance imaging (MRI), positron emission tomography (PET), lymphoscintigraphy (LS), fine-needle aspiration cytology (FNAC), and SLNB.27, 40-42 Both palpation and imaging assessments often lack sufficient accuracy for early LNM detection in OSCC.43, 44 Liao et al. conducted a comprehensive meta-analysis that scrutinized the accuracy of different imaging modalities in detecting cervical LNM in patients clinically diagnosed with cN0 head and neck squamous cell carcinoma (HNSCC). The results showed the following sensitivities and specificities: US revealed a sensitivity of 66% and a specificity of 78%; CT demonstrated a sensitivity of 52% and a specificity of 93%; MRI offered a sensitivity of 65% and a specificity of 81%; and PET provided a sensitivity of 66% and a specificity of 87%. Crucially, the study elucidated that there were no significant differences in the diagnostic performance of these imaging modalities in assessing cervical LNM.43 Another separate meta-analysis conveyed that FNAC exhibited a sensitivity of 69.1% and a specificity of 84.2% in diagnosing cervical LNM, findings that align closely with the previously mentioned imaging modalities.45 LS was reported to hold a sensitivity of 100.0%, a specificity of 81.25%, and an accuracy of 96.66% in the preoperative detection of cervical LNM in patients with OSCC.46 However, it should be noted that this study was predicated on relatively small sample size, a factor that may potentially introduce significant bias.
SLNB, a surgical procedure involving the removal and examination of the first echelon nodal basin, or sentinel lymph node(s), to which a primary tumour drains,47 has been recommended by the NCCN as an alternative to elective neck dissection for identifying occult cervical metastasis in early OSCC.9 Although SLNB has demonstrated higher sensitivity (75%−100%), its technical demands and reliance on expertise and experience warrant caution when considering it as a replacement for elective neck dissection.9, 48, 49
4.2 Neck dissection
Neck dissections, routine surgical procedures for examining neck lymph nodes, serve as preventive measures against occult LNM and are the preferred treatment for LNM in OSCC, excluding extremely advanced, unresectable cancers.4 A prospective randomized controlled trial showed that for early-stage OSCC patients with negative neck, prophylactic neck dissection resulted in higher overall survival and disease-free survival rates compared to therapeutic neck dissection performed after the discovery of neck metastasis through observation.50 This is why the CLNs preservation strategy for OSCC has been rarely mentioned prior to the remarkable discoveries in cancer immunotherapy.
Neck dissections can be categorized into four types: radical, modified radical, selective (with specific notation of the levels or sublevels removed), and extended neck dissections.51 RND is typically used for late-stage patients and is no longer considered the ‘gold standard’ today.52 SND involves selective removal of one or more nodal levels and is primarily used for patients with an N0 neck, although it has been utilized in certain cases to manage an N+ neck..53-55 The Anderson Cancer Center reported that the rates of postoperative neck recurrence in tongue cancer patients who underwent SND and RND were 6.2% and 4.0%, respectively. These data indicate that both neck dissection methods are effective in controlling local recurrence.56 Several studies have shown that the preservation of more anatomical structures in the neck, such as the sternocleidomastoid muscle and the spinal accessory nerve, coupled with reducing the levels of neck dissection, can significantly improve the postoperative quality of life for patients.57, 58 Based on these insights, modern SND, compared to conventional RND, appears to confer more postoperative advantages due to reduced tissue loss. In essence, the saying ‘less is more’ aptly encapsulates these advances.
In OSCC, SND should generally involve the frequently affected lymph node levels of I, II and III, also known as supraomohyoid neck dissection. Data suggest that cancer at the floor of the mouth or anterolateral tongue lesions may carry an elevated risk of level IV involvement, and metastasis may occur at level I–IV in an N+ neck.34, 59, 60 In such cases, SND should be extended to level I-IV. For lesions located in the midline, bilateral SND I-III should be considered.
Recent debates have questioned the necessity of level IIB lymph node dissections due to the low probability of OSCC metastasizing to this area (approximately 6%) and the potential to induce temporary, or occasionally permanent, shoulder dysfunction and disability.61 However, in instances where clinical or pathological evidence confirms metastasis in other areas, dissection of level IIB should still be prioritized. The decision between in-continuity and discontinuous dissections remains contentious for tongue and floor of mouth cancers. In-continuity neck lymph node dissection involves simultaneous removal of the lymph nodes and the primary tumour, mitigating the risk of residual tumour cells in the lymphatic channels between the floor of the mouth and the neck hence reducing locoregional recurrence.62 This procedure, however, necessitates the removal of floor of mouth tissue separating the oral cavity from the neck, potentially affecting tongue movement, swallowing, increasing the risk of postoperative oral neck fistula, and prolonging healing time.63 A meta-analysis suggests early-stage floor of mouth cancers are better served by discontinuous neck dissection, while in-continuity neck dissection is recommended for locally advanced floor of mouth cancer patients.62 For patients positive for clinical lymph nodes who require modified radical neck dissection, the necessity of level V dissection remains disputed. A 2017 meta-analysis revealed that occult metastasis of level V lymph nodes is rare and preservation may be considered when level V is clinically uninvolved.64
Given the life-threatening nature of OSCC, postoperative complications following successful neck dissection are deemed acceptable. However, neck dissection is a complex and challenging surgery. Its impact on patient quality of life post-surgery may be considerably more significant than theoretically predicted, varying with different medical conditions and patient circumstances. Moreover, the removal of a substantial number of CLNs might lead to decreased lymphatic and immune function due to low lymph node volume, a consequence that is challenging to predict. In the era of precision medicine, we advocate for an individualized neck dissection strategy that takes into account the patient's disease condition, general health status, neck anatomy, personal preferences, and existing medical conditions, rather than a strict adherence to traditional neck dissection classifications.
4.3 Association between systemic therapy and CLNs
The term ‘systemic therapy’ typically encompasses chemotherapy and immunotherapy treatments, which are progressively becoming vital in the management of various cancers. For patients with positive resection margins and nodal metastases with extracapsular extension, adjuvant combined chemotherapy and radiation therapy have shown to improve survival rates.65
The initial approval of immune checkpoint blockade therapies, specifically anti-PD-1 and anti-CTLA4 agents, for the treatment of advanced melanoma patients marked a significant milestone in oncology. Subsequent to this approval, these therapies have been expanded and applied to a wide range of cancers, leading to a substantial reshaping of the broader landscape of cancer treatment. The introduction of immune checkpoint blockade therapies has indisputably revolutionized the algorithm of cancer treatment, significantly impacting the management strategy for a variety of cancers, including HNSCCs.66 While immunotherapy has emerged as an integral element of the treatment paradigm for recurrent and metastatic HNSCCs, the benefits of combining immunotherapy with curative radiotherapy or chemoradiotherapy have not yet to prove unequivocally advantageous for patient outcomes.67-69
In recent years, the emergence neoadjuvant therapy has heralded a new era for non-surgical OSCC treatment. Multiple studies suggest that neoadjuvant chemotherapy may play a role in preserving organs and treating borderline resectable OSCC.70, 71 Promising findings from several studies have evidenced the safety and efficacy of neoadjuvant immunotherapy in the treatment of HNSCCs.72-75 However, it is crucial to note that the term HNSCC is an umbrella term that not only encompasses OSCC but also malignancies in other locations, such as laryngeal, nasopharyngeal, esophageal, thyroid cancers, among other malignant tumours in the region. Clinical trials focusing on HNSCCs frequently encompass a limited representation of patients with OSCC, and there is a scarcity of investigations specifically exploring lymph node associations with immunotherapy. A phase 2 randomized clinical trial demonstrated that neoadjuvant immunotherapy for OSCC can achieve significant clinical to pathologic downstaging.76 This study also found that of 15 patients, 14 showed increased max standardized uptake values in PET-CT examinations but no pathological invasion, indicating the immunotherapy-induced effect. However, research exploring the use of systemic therapy for LNM in OSCC is currently limited. Given the significant efficacy of neoadjuvant immunotherapy for primary cancer, it should likewise be effective for LNM, although further clinical trials are required for verification.
As significant immune organs, lymph nodes hold substantial implications for tumour immunotherapy in this new era. Preoperative pharmaceutical intervention provides an opportunity for researchers to analyze excised lymph nodes, thereby advancing our understanding of their role in immunotherapy. It is increasingly accepted that the tumour-draining lymph node (TDLN) is a dynamic, multifaceted entity that fosters metastasis while hindering immune surveillance. These changes stem from the dysregulation of inherent leukocyte activity and stromal remodeling, which creates a milieu favoring immune suppression, even in the presence of tumour-specific antigens.77 Targeting the TDLN could therefore be a pivotal immunotherapeutic approach, given its potential as a reservoir of tumour-derived antigens requiring immune stimulation. The journal Cell recently published a significant finding: maintaining lymph node integrity until the completion of immunotherapy can enhance its efficacy against tumours.7 This groundbreaking discovery underscores the importance of preoperative immunotherapy and suggests that preserving uninvolved lymph nodes until immunotherapy is completed might be a consideration.
4.4 Association between radiotherapy and CLNs
Surgery has become the gold standard for neck management in OSCC patients. Currently, only those patients who are unsuitable for, or unwilling to undergo, surgery will consider radical radiotherapy as a neck treatment.78 Since 2009, intensity-modulated radiation therapy (IMRT) has progressively replaced traditional radiation therapy for neck LNM. As the most precise radiation therapy technique, IMRT delivers smallest possible dose and radiation field to achieve optimal clinical outcomes.79 Radiotherapy has the potential to inflict damage on small blood vessels and fibroblasts, thereby interfering with typical processes of proliferation and remodeling. This disruption consequently threatens tissue healing and regeneration. Implementing a neck dissection following radiotherapy or chemoradiotherapy significantly increases the likelihood and severity of surgical site complications.80 For now, neck irradiation frequently serves as adjuvant treatment after surgery. Its primary objective is either to inhibit local recurrence after surgery or to eradicate potential microscopic local metastases.
Some recent studies have demonstrated that elective neck irradiation yields similar results to prophylactic neck dissection.81 Given the scarcity of experienced surgeons capable of performing neck dissection and the extensive training required, this radiotherapy approach could help alleviate healthcare system pressures. It also represents a worthwhile alternative treatment option that maximizes the preservation of lymph nodes. There is no current consensus on the appropriate use of postoperative radiotherapy (PORT) for pathological N1 (pN1) stage patients. Some experts may advise against PORT if there are only two positive nodes, but it is generally recommended for patients with three or more nodes or evidence of extracapsular spread.82 A recent multicenter prospective cohort study has incited reconsideration of the blanket recommendation of adjuvant radiation therapy for all pN1 OSCC patients. The study found that while adjuvant radiation therapy can reduce the risk of recurrence within a 5-year span for pT1/2 pN1-OSCC, it does not significantly influence the overall 5-year survival rate. Furthermore, it notably decreases the postoperative quality of life for patients.83
4.5 ‘PRECISE’ model for neck management
The administration of radiation therapy or systemic therapy could induce a range of complications, including oral mucositis, radiation dermatitis, dysphagia, hyposalivation and xerostomia, taste alterations, trismus, soft tissue necrosis and osteoradionecrosis, head and neck lymphedema, and voice and speech alterations.84 To enhance treatment outcomes while simultaneously reducing cost, precision medicine is considered the future direction. We recommend that surgeons consider the ‘PRECISE’ model when addressing this issue (Figure 6). In confronting the challenge of cervical LNM in OSCC, we need to shift away from treating symptoms rather than the root cause, and place greater emphasis on primary cancer prevention to fundamentally reduce the incidence of LNM.
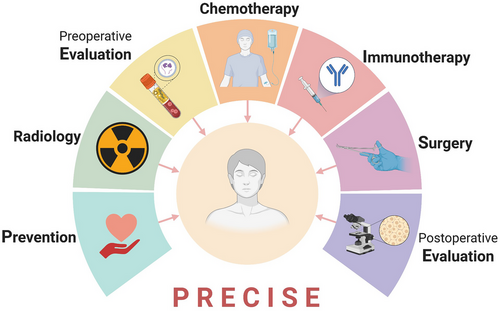
‘P’ corresponds to prevention, which involves educating individuals to amend unhealthy lifestyle habits such as tobacco, alcohol, and betel quid consumption, identified as clear risk factors of OSCC. This will reduce the probability of cancer development. ‘R’ symbolizes radiology, encompassing radiological examination and radiation therapy. The former is an essential procedure to ascertain the clinical TNM stage of the tumour, providing a foundation for subsequent examination and treatment plans. For patients exhibiting positive lymph nodes post-surgery, adjuvant radiation therapy is often suggested. The first ‘E’ refers to preoperative evaluation, necessitating a comprehensive assessment of the patient's condition, based on thorough examination, to devise a personalized neck treatment plan. Alongside routine preoperative examinations to confirm diagnosis and exclude surgical contraindications, biomarker testing should also be performed for patients considering neoadjuvant therapy. This is to assess the anticipated response and screen precise therapeutic targets if available. ‘CI’ pertains to chemotherapy and immunotherapy, optional neoadjuvant therapies for patients with locally advanced-stage disease. ‘S’ denotes surgery, where individualized strategies are recommended. The final ‘E’ represents postoperative evaluation, which, based on pathological TNM staging and follow-up results, determines whether adjuvant therapy or additional surgery is required. Additionally, evaluating pathological and molecular indicators of intraoperative excised lymph nodes in the context of neoadjuvant therapy can further advance the application of precision medicine in neck management.
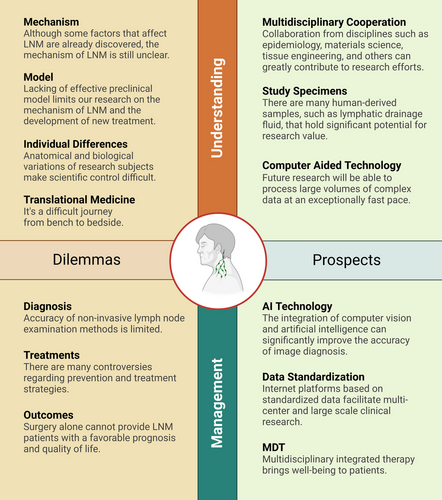
5 DILEMMAS AND PROSPECTS
5.1 Preoperative assessment for CLNs
There is currently no sufficiently accurate non-invasive method to evaluate CLN status. Some patients with clinically positive lymph nodes may show negative results in lymph node pathology examinations, such as in Castleman's disease.85 This limitation of technology creates an uncertainty for CLNs akin to Schrodinger's cat, where metastasis is hard to determine before lymph nodes are removed and examined histopathologically. Emerging non-invasive methods for assessing CLNs primarily include artificial intelligence-assisted predictive models,86, 87 nanoparticle-based lymphatic system imaging,88 and biomarker-based liquid biopsies.89 These studies are still in the experimental stage, and their widespread clinical application remains a distant goal.
5.2 Neck dissection under the concept of precision medicine
The balance between curative and function-preserving approaches remains a controversial topic in cancer surgery. LNM in OSCC leads to a high mortality rate due to the unique anatomy of the neck. Historically, more aggressive approaches have often been taken for CLN management. However, the development of vascularized free flap transplantation techniques has made it possible to preserve neck vessels, giving patients hope for reconstruction and functional restoration, thereby improving their postoperative quality of life. Recent studies highlight the importance of immune cells in regional lymph nodes for OSCC immunotherapy.7, 90 Future surgical considerations may include preserving lymph nodes not invaded by the tumour, whenever possible.
The most recognized and broadly utilized staging system for OSCC is the TNM staging system.5 While it considers factors like lymph node size and laterality, it does not emphasize the significance of the total number of nodes containing metastases. A retrospective study by Ho et al. established the number of metastatic lymph nodes as an important predictor of OSCC mortality, carrying greater prognostic value than other factors like lymph node size and contralateral involvement.91 They proposed a novel nodal staging system with superior concordance than the 8th edition of AJCC system, offering a concise model for better differentiation and identification of high-risk patients, potentially assisting in the selection of locally advanced patients requiring neoadjuvant therapy. In the precision medicine era, targeted immunotherapy offers new hope for locally advanced OSCC treatment. Accordingly, more precise clinical staging of the disease should be proposed to guide non-surgical treatment approaches better.
Technologies like indocyanine green or radiotracer-based methods are currently being explored for real-time visualization of lymph nodes during surgery, aiming to perform more precise lymph node dissection.92 However, these technologies have low specificity in identifying metastatic cancer in lymph nodes. To achieve precise removal of metastatic lymph nodes, further exploration is required in the application of related nanoparticles and multi-technology combinations. Robotic-assisted surgery provides a more minimally invasive approach to neck dissection, leading to better cosmetic outcomes.93 However, the issues of longer operative times and higher costs still need to be further addressed in robotic-assisted neck dissection.
To this day, further clinical research remains necessary to address queries concerning the patterns of cervical LNM, neck management strategies for OSCC patients, the scope and timing of neck dissection surgery, and multidisciplinary treatment approaches for patients with LNM. Electronic data capture (EDC) systems, based on internet cloud platforms, have the potential to greatly simplify the complexity of multi-center, large-scale clinical research.94 Despite the era of big data, significant untapped potential exists within the clinical data generated during diagnosis and treatment. Yet, currently, there is considerable heterogeneity in the documentation of medical records and clinical data across global institutions. Advancing medical data standardization could further foster development in clinical research.
5.3 The role of TDLNs in immunotherapy for OSCC
A preclinical animal study demonstrated that TDLNs play a crucial role in the initiation and expansion of anti-tumour immunity during early stages. However, once immune memory is established, anti-tumour immunity ceases to rely on TDLNs. Radiation therapy solely targeting the primary tumour can enhance the effectiveness of immunotherapy, while selective radiation targeting the lymph nodes can mitgate this effect.95 Saddawi et al. conducted an additional preclinical study probing the role of TDLNs in immune checkpoint inhibitor (ICI) therapy. Employing a murine model in this experiment, they observed that the surgical removal of lymph nodes prior to ICI therapy significantly decreased overall survival rates. Further exploration reveals TDLNs’ key role in coordinating antigen-specific CD8-driven immunity within the tumour immune microenvironment, and their harboring of a specific population of conventional type I dendritic cells pivotal in the response to ICIs. The administration of ICIs prior to neck dissection could potentially enhance primary tumour treatment responses and promote immunosurveillance, offering protection against locoregional nodal metastasis. Additionally, delayed neck dissection following ICI therapy could mobilize peripheral anti-tumour immunity.96
Both studies affirm the crucial role of TDLNs in tumour immunotherapy. Nonetheless, additional research is required to elucidate the precise timing of TDLN functionality and the optimal timing for the selection of immunotherapy and surgical treatment, thereby deepening our understanding of this facet.
5.4 Research on LNM
Since the 19th century, considerable strides have been made in understanding cervical LNM in OSCC. However, the general patterns and underlying molecular mechanisms remain elusive.
Over may years, scholars have reported OSCC metastasis rates from various primary sites to different CLN levels based on retrospective studies. Yet, these studies’ conclusions are not consistently aligned. To delve deeper into the general patterns of LNM in OSCC, there is a need for systematic collection and summary of global studies on OSCC's LNM rate. This approach, based on comprehensive data retrieval and extraction, aims to yield more scientifically valuable insights.
Historically, LNM studies in OSCC have predominantly concentrated on the genetic and cellular levels. Recent research posits that the lymph node is more than a passive staging area during metastasis; it is a crucial site for initiating systemic immunosuppression.77 Consequently, in the burgeoning era of tumour immunotherapy, it is crucial to establish effective in vivo models such as mice,97 zebrafish,98 or rabbits.99 Regarding in vitro research, the limitations of 2D cell culture models have driven the development of 3D models, which better mimic the natural tissue's cellular microenvironment. Spheroids, organoids, and 3D bioprinting are commonly used methods for creating 3D models, with substantial potential for studying LNM.100 Furthermore, the Organs-on-Chips (OoCs) system is an emerging tool for in vitro experiments, capable of simulating human organ functions and disease pathophysiology.101 These perfused microfluidic chips simulate the tumour microenvironment and host cells and tissues similarly to in vivo conditions, thereby enabling a profound understanding of human cancer's pathophysiology in an organ-related context while circumventing ethical issues.102 Although several lymph node on-chips have been developed, this area warrants further exploration in the OSCC field.103
Nanotechnology-mediated drug delivery systems have been proposed as a potent approach for combating cancer metastasis. Lymph nodes targeted treatment will be a focal point in future LNM treatments. For instance, Liu et al. introduced a system named iCluster, which can target nanoparticles from primary tumours to potential metastatic lymph nodes via tumour lymphatic vessels, thereby inhibiting metastasis.104 There is a demand for more research in the OSCC LNM domain.
EVs are a heterogeneous group of membrane-bound vesicles released by cells, isolated from various types of body fluids such as blood, saliva, pleural effusion, and ascites.105 Wang et al. discovered that EVs-loaded laminin-332 could serve as a diagnostic biomarker for LNM in OSCC patients.106 Our team's recent research confirmed the enrichment of EVs in the postoperative drainage fluid of OSCC patients and discovered two metabolic proteins, EHD2 and CAVIN1, to be strongly correlated with LNM. This discovery has paved the new way for studying the relationship between EVs and LNM in OSCC through postoperative drainage fluid.107 However, there is still a long way to go for the clinical translation of these laboratory findings. Single-cell RNA sequencing (scRNA-seq) and tissue microarray have proven beneficial for better understanding the heterogeneity and connections between metastatic lymph nodes and primary OSCC.108, 109 However, current research has not yet comprehensively and systematically elucidated the patterns of LNM occurrence and development in OSCC. Despite being a vital immune organ, lymph nodes’ specific immune functions, particularly their physiological roles in anti-tumour immunity, still harbor numerous unsolved mysteries. Given the potential involvement of lymph nodes in complex immune changes during OSCC's development and progression, it is timely to further investigate the molecular and biological connections and changes between CLNs and primary cancer.
Current studies on OSCC LNM often focus on a specific stage of metastasis. However, increasing evidence suggests that LNM is a dynamic process. Spatial transcriptomics (ST), a technique capable of capturing and detecting transcriptomes with spatial resolution, has emerged as a formidable tool for systematically exploring biological systems.110 Leveraging scRNA-seq for ST enables continuous monitoring of target cell characteristics during various stages of cell colonization, migration, differentiation, and phenotypic transitions.111 Future research could benefit from placing increased emphasis on ST analysis in probing the underlying mechanisms of LNM in OSCC. This approach could provide insights into the dynamic patterns of lymph node involvement.
Overall, numerous unresolved issues persist regarding OSCC LNM from both clinical and fundamental research perspectives. As emerging technologies continue to evolve, they may provide solutions to these challenges. Figure 7 encapsulates the dilemmas and prospects we encounter in our quest to understand and address LNM in OSCC.
6 CONCLUSION
Over the past two centuries, our comprehension and management of LNM in OSCC have been continually evolving. The trajectory of this development has been towards the discovery of increasingly precise and personalized treatment methods, with the aim of reducing patient trauma and enhancing both survival duration and quality of life. This approach can be encapsulated by the adage, ‘less is more’ (Figure 8).
Recent research on the role of lymph nodes in tumour immunity has challenged traditional approaches to managing CLNs in OSCC. Considering that LNM is a clear prognostic indicator of poor outcome, it has been suggested that TDLNs should be prophylactically removed. However, the removal, while potentially curbing metastasis, also eliminates an important reservoir of potential anti-tumour immune cells, thereby undermining tumour immunotherapy strategies.
Addressing this catch-22 situation necessitates further research focusing on the role of lymph nodes in cancer immunotherapy, as well as the interactions between OSCC and CLNs. This nuanced understanding will allow for the development of more effective and less invasive treatment strategies, thereby promoting improved patient outcomes. That is precisely the area of focus and pursuit in clinical and translational medicine.112
AUTHOR CONTRIBUTIONS
Conceptualization, B. L. and L.-L. B.; writing—original draft preparation, L.-M. C., N.-N. Z., Z.-Z. L., and F.-Y. H.; writing—review and editing, N.-N. Z., Z.-Z. L., B. L., Y. X., and L.-L. B.; figure drawing, L.-M. C.; funding acquisition, L.-L. B. All authors have read and agreed to the published version of the manuscript.
ACKNOWLEDGEMENTS
The Figures 1, 2, 5, 6, 7 and 8 were created on biorender.com. Thanks for the powerful function and exquisite materials provided on their website. This study was supported by Postdoctoral Science Foundation of China (grant numbers: 2018M630883 and 2019T120688), Hubei Province Chinese Medicine Research Project (grant number: ZY2023Q015), Natural Science Foundation of Hubei Province and Wuhan Young Medical Talents Training Project to L.-L. Bu.
FUNDING INFORMATION
Postdoctoral Science Foundation of China, Grant Numbers: 2018M630883 and 2019T120688; Hubei Province Chinese Medicine Research Project, Grant Number: ZY2023Q015; Natural Science Foundation of Hubei Province and Wuhan Young Medical Talents Training Project
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS APPROVAL
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
No data were used for the research described in the article.




