The immunosuppression effects of deforolimus (ridaforolimus, AP23573) on allograft organ transplantation
The work was done in the Organ Transplantation Institution (OTI), medical college of Xiamen University, Xiamen, China.
Abstract
Objects
We conducted this study to confirm the immunosuppression effect of Deforolimus in heart allotransplantation and find its mechanism.
Material and methods
We used vitro methods to confirm the immunosuppression of Deforolimus in T cell subgroups. Then, we conducted heart allotransplantation from BALB/c donors to C57BL/6 recipients with the oral administration of Deforolimus or blank solvent to contrast the immunosuppression effect in vivo. We used the flow cytometry(), clone anergy test, ELISA (enzyme-linked immunosorbent assay), apoptosis test, and lymphocyte translation (LT) to find the mechanism of Deforolimus’ immunosuppressive action.
Results
Studies in vitro demonstrated that Deforolimus had immunosuppressive action. Studies in vivo demonstrated that Deforolimus could inhibit the immune system of the heart allotransplantation recipients to extend the recipients’ life. The mechanisms of Deforolimus’ immunosuppressive action included induction of Tregs (regulatory T cells), induction of T cells anergy, and decreasing proportion of T cells in spleens and lymph nodes.
Conclusion
Deforolimus suppressed the immunological rejection of mouse cardiac allotransplantation which laid the foundation of Deforolimus applying to solid organ allotransplantation.
1 INTRODUCTION
Organ transplantation is one of the best methods to address end-edge organ failure, and heart transplantation has been applied to its clinical field, leading to good effects. However, one of the biggest problems in the application is the recipients’ immune rejection, which leads to premature failure of the transplanted organs and a series of physiological problems that make the recipients unable to live a normal life. In order to extend the lives of patients who have undertaken organ transplantation, a variety of immune inhibitors have been applied. However, general immunosuppressive methods suppressed the immune system of patients, meanwhile, increased the tumor incidence of patients, which hindered their clinical application.1, 2 Early work had indicated that the first-generation of mTOR (mammalian target of rapamycin) inhibitor, rapamycin both had immunosuppressive and antitumor activity with low nephrotoxicity. This had been clinically accepted and indicated that mTOR was a potential target for cancer therapies.3-8
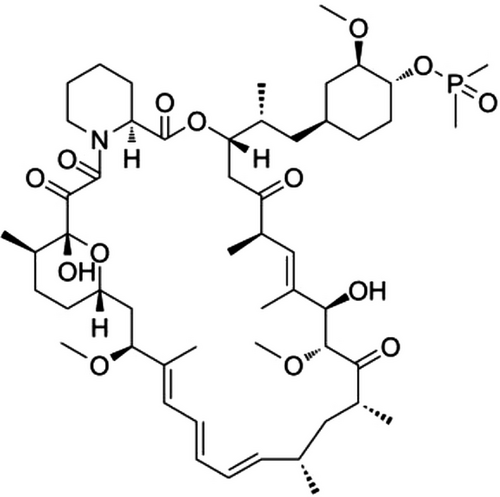
One of rapamycin derivatives, Deforolimus (ridaforolimus, AP23573), has better aqueous solubility, affinity and stability than that of rapamycin.9, 10 The structure of Deforolimus was showed in Figure 1. A large amount of phase I, phase II and phase III clinical trials had been published, which revealed its effect of tumor inhibition.11-15 However, there was no report about the immunosuppression of Deforolimus in organ transplantation. In order to find the immunosuppression of Deforolimus in the field of organ transplantation, we conducted this study. We aimed at confirming the immunosuppression effect of Deforolimus and find the mechanism, intending to lay a foundation for its clinical trials in organ allotransplantation.
2 RESULTS
2.1 Deforolimus inhibits the proliferation of lymphocytes in vitro
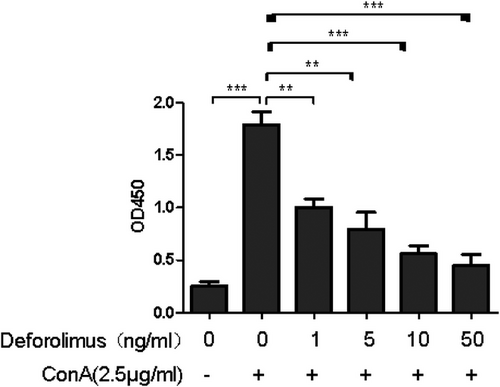
In order to determine whether Deforolimus has potential of inhibition, we conducted lymphocyte transformation test with the addition of Deforolimus. The result in Figure 2 revealed that cells were stimulated by the addition of Con A, and the stimulated cells were inhibited by Deforolimus (**p < .01). With the increasing concentration of Deforolimus (0, 1, 5, 10, 50 ng/mL), the stimulated cells were inhibited more (**p < .01; ***p < .001).
2.2 Deforolimus prolonged recipients’ survival time and protected the allogeneic cardiac grafts from inflammatory cell infiltration
Adopting the optimal oral dose (5 mg/kg), we conducted heart allotransplantation from BALB/c to C57BL/6 with oral administration of Deforolimus (5 mg/kg) or normal saline (control group) from d0 (the day of operation) until graft rejection. Figure 3A shows that the 50% survival time of Def (Deforolimus) group was 17.5d and that of the Con (control) group was 8d (p = .0032). Body weight (BW) changes in Figure 3B shows that the mean BW of mice in Con group restored on the third day when the mean BW of mice in Def restored at the eighth day after allotransplantation.
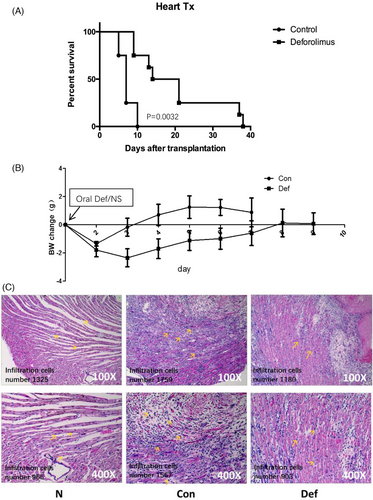
For searching how Deforolimus extended the survival time of cardiac graft, we took out the grafts on the 8th day after transplantation with anesthesia and performed H&E staining. The results were shown in Figure 3C. The inflammatory cell number was counted by Image J (Enhance contrast 6.0%, Noise tolerance13). In group N (naïve), the inflammatory cell counts were 1325 and 965 under 100-fold and 400-fold microscope separately; in group C (control), the inflammatory cell counts were 1759 and 1567 under 100-fold and 400-fold microscope separately; in group D (Deforolimus), the inflammatory cell counts were 1180 and 903 under 100-fold and 400-fold microscope separately. The figures represented six separate experiments.
2.3 Deforolimus reduced the level of immunity in heart transplant recipients
We detected level of several factors on the 8th day post-transplantation. As Figure 4A showed, the spleen of control group was 2 cm in length when that of Def group was 1.5 cm. The results represented three separate experiments.
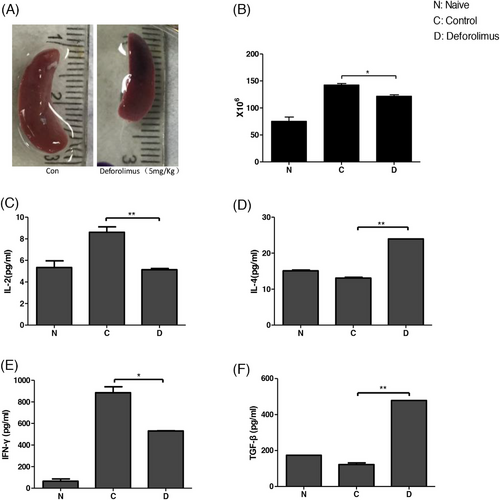
We counted the cell number of spleen; the result was shown in Figure 4B. The data showed mean ± SD and represented three separate experiments (*p < .05). The mean entire spleen cell number of naïve B6 mice was 7.5 × 107 when that of Con group was 1.4 × 108, and Def group was 1.25 × 108 (*p < .05). Heart allotransplantation stimulated the immunity of recipients, and Deforolimus inhibited the level of immunity. The difference was significant.
We tested the serotype cytokine concentrations by enzyme-linked immunosorbent assay (ELISA). The results were shown in Figure 4C-–F. Deforolimus reduced the secretion of cytokine IL-2 and IFN-γ (**p < .01; *p < .05 separately) and increased the secretion of cytokine TGF-β (transforming growth factor-β) and IL-4(**p < .01; **p < .01 separately).
2.4 Deforolimus changed different lymphocytes’ proportion
In order to study whether Deforolimus protected allogenic grafts by clonal deletion or not, we tested the proportion of Th (helper T cell), Tc (cytotoxic T cell), and B cells in the spleens and lymph nodes at the 8th day post-transplantation. The results were shown in Figure 5A, Deforolimus reduced the proportion of CD4+T cells and CD8+T cells of spleens and lymph nodes. At the same time, Deforolimus increased the proportion of B lymphocytes of spleens and lymph nodes. Thus, clonal deletion was one of the mechanisms that Deforolimus reduced immune cells infiltration and prolonged survival time of allografts.

Regarding immune tolerance prolonging graft survival, the study of Tregs was of great significance. We investigated the Tregs proportion changes in spleens of both groups by flow cytometry (FCM). The results were shown in Figure 5B. Deforolimus increased Tregs proportion, indicating that inducing Tregs was one of the mechanisms that Deforolimus induces immune tolerance.
2.5 Deforolimus induced lymphocyte clone anergy but did not cause lymphocytes’ apoptosis
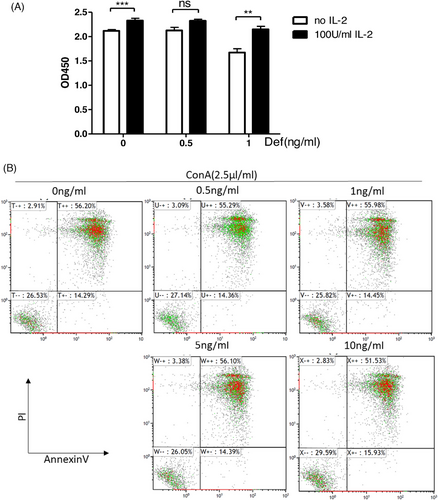
In order to study whether Deforolimus could lead to the clone anergy of T cells or lymphocyte apoptosis, we conducted lymphocyte clone anergy test and T cell apoptosis test. The results were shown in Figure 6A. At the concentration of 1 ng/mL, Deforolimus inhibited T cell proliferation, and the proliferation of T cells was restored with 100 U/mL IL-2. Clonal anergy of T cells was one of the mechanisms that Deforolimus induced immune tolerance. In Figure 6B, with the increasing concentration of Deforolimus (0, 0.5, 1, 5, 10 ng/ml), the apoptosis rates were 56.20%, 55.29%, 55.98%, 56.10%, and 51.53%, respectively. Deforolimus could not increase lymphocytes’ apoptosis proportion. So, that inducing lymphocytes’ apoptosis was not the reason why Deforolimus induces immune tolerance.
3 DISCUSSION
As the biggest peripheral immune organ, spleen is the key to the development of immune tolerance.16 It reflected the immunological rejection level. The results showed oral administration of 5 mg/kg Deforolimus could prolong the survival time of grafts with weaker immunological rejection and inhibited the immune system of recipients after cardiac transplantation.
T-cell-mediated immunosuppression was one of the primary mechanisms responsible for maintaining transplantation tolerance in vivo.17 CD4+T cells and CD8+ T cells played a critical role in allograft rejection and graft failure.18, 19 Both IL-2 and IFN-γ were cytokines of Th1 cells in tumor rejection. The increasing IL-4 indicated increasing expression of Th2 cells.20, 21 The ELISA results showed that Deforolimus increased expression of Th2 cells and decreased expression of Th1. As Th1 cells expressed less and the Th2 cells expressed more, the Th1/Th2 ratio reversed.22-24 Regulating the ratio of Th1/Th2 is one of the mechanisms of immune regulation.25, 26 We speculated that it was one of the mechanisms through, which Deforolimus inhibited graft rejection. Tregs played an important role in the process of inducing transplantation immune tolerance by inhibiting the activation and proliferation of other immune effector cells.27, 28 TGF-β induced Foxp3-positive Tregs (iTreg) and inhibited the proliferation of T cells via Foxp3-dependent and independent mechanisms as well as cytokine production.29, 30 Research showed that Breg-mediated tolerance was also dependent on TGF-β.31 In this study, we found that Deforolimus increased the Treg proportion in spleens. Thus, inducing Tregs was one of the mechanisms that Deforolimus inhibited the immune system of heart transplantation recipients. Clone anergy method proved it further. IL-2 was an essential factor for the generation, survival, stability, and function of Treg.32, 33 The addition of IL-2 restored T cell proliferation, and Treg cells mediated suppression by inhibiting IL-2.34, 35 Clonal anergy was deemed as mechanisms to balance B cell tolerance and immunity.36 Meanwhile, it was one of the mechanisms that T helper cell induced immune tolerance.37 The molecular surface marker of CD19 is present in pre-B lymphocytes and immature B lymphocytes.
This study on Deforolimus in heart allotransplantation model opened a new sight in the field of organ transplantation. Since the 1990s, public organ donation movement has developed. We have successfully improved the registration rate of organ donors, which has stimulated the activity of organ transplantation from person-to-person.38 Experiments have shown that rapamycin (2 mg/kg/day, intraperitoneal injection) in mouse models of allogeneic heterotopic heart transplantation may extend graft survival to up to 20 days42, which was shorter than that of Deforolim
Limitations: We didn't set a control group of rapamycin, which was error in experimental design. If there was a control group of rapamycin, the results would be more convincing, as immunosuppression effect of rapamycin has been widely accepted in clinical. Oral Deforolimus schedule (5 mg/kg·d, 5 consecutive days and 2 days off) of Def group mice after transplantation was supposed to be more reasonable, as the half-life of Deforolimus (∼50 h) was long. In spite of this, this study lays a foundation for the application of Deforolimus in human-to-human heart transplantation. Deforolimus could hopefully be applied in the field of clinical heart transplantation.
4 MATERIALS AND METHODS
4.1 Animals
In this study, C57BL/6 female mice (H-2b) were 8 to 12 weeks, weighing 20−23 g. BALB/c male mice (H-2d) weight 20−23 g. All animals were purchased from the Chinese Academy of Sciences, Shanghai SLAC Laboratory Animal Co., Ltd. (Shanghai, China) and raised in a specific-pathogen-free environment.
4.2 Drugs
Deforolimus was provided by the Fujian Institute of Microbiology (Fuzhou, Fujian Province, China). The molecular structure of the compound was shown in Figure 1.
4.3 Heart allotransplantation
Heart allotransplantation was operated from BALB/c donors to C57BL/6 recipients by vascularized heterotopic operation, as Ma et al.39 described. Animal experiments’ implementation was in accordance with the Institutional Animal Care and Use Committee and Animal Ethics Committee. Before operation or tests, donors and recipients were anesthetized to reduce suffering. Anastomosed vessels from the neck and donor heart by a non-suture cuff technique were performed as described previously.40 Successful operation was judged by palpation of powerful donor heartbeat. Graft survival was monitored (daily) by palpation, and body weight was recorded (daily) until the donor heart death. Recipients (n = 12) were given oral Deforolimus (5 mg/kg·d, n = 6) or blank solvent (n = 6) daily from the day of transplant operation (d0) to graft death.
4.4 Lymphocyte isolation
Spleens were taken from heart recipients at d8 after operation. Then, ground the spleens into pieces gently separately in 4 mL of red cell lysate. Five minutes later, the dissociation was terminated with 10 mL of phosphate-buffered saline (PBS) solution. Then, we filtrated the mixture with nylon membrane. Removed the supernatant after centrifugation at 1600 rpm for 5 min. The swill and centrifugation were repeated once as above for cleaning the impurity. Then, swilled the cells in 2 mL of RPMI 1640 medium (Hyclone, Thermo Fisher, USA) containing 10% fetal bovine serum (FBS, Gibico), 0.1% antibiosis (Solarbio). Cell counter counted.
4.5 T lymphocyte isolation
T lymphocytes were separated from the isolated lymphocytes, as section 4.4 described. Note that 0.25-g nylon wool was taken and soaked in PBS for 5 min, then cleaned the nylon wool with RPMI 1640 medium (Hyclone) until the nylon wool was completely immersed. Suspended 5 × 107 lymphocytes in RPMI 1640 medium until the cells dispersed evenly in nylon wool. Then incubated the nylon wool with 5 × 107 lymphocytes in 37°C, 5% CO2 atmosphere for 45 min to 1 h. Washed the nylon wool with 10 mL of 1640 complete medium, and collected the eluent. Centrifugalized the eluent at 1600 rpm, 5 min and reserved the sediment which were T cells. We suspended the sediment in complete RPMI 1640 mediums (RPMI 1640 medium, 10% FBS, 0.1% antibiosis). Counted the cells. The flow analysis showed that the purity of T cell ([CD3]-labeled) was above 85%.
4.6 Lymphocyte transformation test
Plated 1 × 106 cells/well from B6 (female mice [H-2b], 8 to 12 weeks) spleen lymphocytes in a 96-well plate, with the addition of 5 μg/mL Con A (Sigma) and gradient concentrations of Deforolimus (0, 1, 5, 10, 50 ng/mL) to each 200 μL volume reaction well. Each element had three parallel wells. Cells cultured with Con A and no Deforolimus were used as negative controls. Cells cultured without Con A or Deforolimus were used as blank groups. Cells cultured with Con A and Deforolimus were used as experimental groups. Cultured the reaction plate in 37°C, 5% CO2 humidified atmosphere for 48 h. Cell proliferation ratio was detected through ELISA, BrdU (colorimetric) immunoassay (Millipore, USA) according to the manufacturer's instructions. The absorbance of 450-nm wavelength was detected in reference to the absorbance at 630 nm using the microplate reader (BIO-RAD, USA), which reflected its cell number. The inhibition level was calculated with respect to control groups.
4.7 Graft histologic analysis
Graft was collected at d8 (median survival time) after heart transplantation. Then, a slice (5-mm thick) of each sample was cut for histological evaluation. Paraffin-embedded tissue sections (5-μm thick) were stained with hematoxylin and eosin, following the previous procedure. Rejection was judged based on the extent of lymphocytic infiltration.
4.8 Cytokine analysis through ELISA
Blood serum was gathered from the heart transplantation recipients (n = 6) on the 8th day post-transplantation. Mouse ELISA kit (eBioscience) was used to analyze the expression of IL-2 (interleukin-2), IL-4, TGF-β (transforming growth factor-β), and IFN-γ (interferon-γ), according to the manufacturer's instructions. The absorbance of 450-nm wavelength reflected its concentration. The results showed cytokine's mean ± standard deviation (SD) of three wells of each group.
4.9 5, 6- carboxyfluorescein diacetate, succinimidyl ester detection of lymphocyte proliferation
5, 6- carboxyfluorescein diacetate, succinimidyl ester (CFSE) is a type of fluorescent cell staining dye which reflects the proliferation of cells without affecting its apoptosis and death. The more cells division, the weaker fluorescence intensity will be. The B6 spleen lymphocytes were stained with CFSE, according to the manufacturer's instructions. B6 spleen lymphocytes (1 × 106 cells/well) were dyed in a 96-well plate with the addition of 2 × 105 BALB/c spleen lymphocytes and gradient concentrations of Deforolimus (0, 1, 5, 10, 50 ng/mL) to each reaction well. Each element had three parallel wells. After 72 h culture, these cells were stained with surface antibodies (i.e., fluorescein isothiocyanate [FITC], anti-mouse CD4; phycoerythrin [PE], anti-mouse CD8; allophycocyanin [APC], anti-mouse CD19; eBioscience). Subsequently, we use FCM to calculate the cell number.
4.10 Lymph node fetch
Lymph nodes from the groins of heart allotransplantation recipients were obtained. Then, placed the lymph nodes in 5 mL PBS solution and ground it into suspension, with nylon membrane filtration. The obtained solution was the lymph node suspension.
4.11 Flow cytometry detection of Tregs
According to the Mouse Tregs Staining Kit's instructions, we labeled the CD4+CD25+T cells of the B6 heart recipients by PE-anti-mouse Foxp3 molecules (eBioscience). We labeled CD4+T cells by FITC molecules (eBioscience). We use FCM to detect the cell number.
4.12 T cell clone anergy
Two groups were set for the lymphocyte transformation test with the addition of different concentrations of Deforolimus (0, 0.5, 1, ng/mL) to the reaction well and the addition of 100 U/mL IL-2 to one of the groups. The 96-well plate was incubated for 72 h, and cell proliferation ratio was obtained using ELISA, BrdU (colorimetric) immunoassay (Millipore, USA), according to the manufacturer's instructions. The absorbance of 450-nm wavelength was detected in reference to the absorbance at 630 nm using the the microplate reader (BIO-RAD, USA). The inhibition level was calculated with respect to the negative control value.
4.13 T cell apoptosis
Gradient concentrations (0, 0.5,1, 5, 10 ng/mL) of Deforolimus were added to the T lymphocyte transformation plate before incubation for 72 h. After incubation, the cells were gathered separately, and the apoptosis kit was used, following the manufacture's instruction (BD). Samples were stained with Annexin V l and PI staining solution for 10 min, avoiding light, and the samples were tested with FCM after 1 h.
4.14 Statistical analysis
The mean survival time was analysed in Kaplan and Meier's method. We used one-way analysis of variance to compare each group of measurement data (MLR, LT, ELISA) as record.41 We used t-test to analyze the significant difference between grouped data. Two-sided p < .05 (paper marked ‘*’) means a statistic difference, p < .01 (paper marked ‘**’) means a significant difference, p < .001 (paper marked ‘***’) means a very significant difference. Data analysis and mapping were made by GraphPad Prism (GraphPad Software, USA).
ACKNOWLEDGEMENTS
This work was supported by grants from the the National Natural Science Foundation of China (grant number: 81771271), Incubation project of Zhongshan Hospital (Xiamen), Fudan University (grant number: 2020ZSXMYJ03), Xiamen Medical and Health Guidance Project (grant number: 3502Z20224ZD1087). The authors were fully responsible for all content and editorial decisions. The paper was affiliated to Zhongshan Hospital (Xiamen), Fudan University.
CONFLICT OF INTEREST STATEMENT
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Open Research
DATA AVAILABILITY STATEMENT
The data used to support the findings of this study were available from the corresponding author upon request.




