Isovalerylspiramycin I inhibits proliferation, migration and invasion of osteosarcoma cells by targeting Topoisomerase 1 and suppressing the ataxia telangiectasia and Rad3-related/checkpoint kinase 1 pathway
Abstract
Purpose
Topoisomerase 1 (TOP1) plays a crucial role in various cell cycle processes and its dysregulation can lead to the development of multiple tumours. However, conventional TOP1 inhibitors such as topotecan and irinotecan have poor clinical efficacy in osteosarcoma (OS) patients. This is partly due to the activation of the ataxia telangiectasia and Rad3-related/checkpoint kinase 1 (ATR/CHEK1) DNA damage repair pathway, which repairs TOP1 poison-induced DNA lesions, compromises the cytotoxicity of TOP1 inhibitors and contributes to drug resistance. Therefore, there is a need to develop more effective TOP1 inhibitors for OS.
Experimental design
In this study, we evaluated the antitumor effects of isovalerylspiramycin I (ISP-I), a novel macrolide antibiotic, using various assays including CCK-8 proliferation assays, wound healing migration assays, Transwell invasion assays, apoptosis, cell cycle, DNA replication and damage analyses on OS cells. We also performed a surface plasmon resonance-high-performance liquid chromatography-mass spectrometry assay to identify ISP-I's direct target protein in OS. Molecular docking analysis, thermoshift assays, enzyme activity assays and reverse tests were used to confirm ISP-1′s target. Finally, we tested the efficacy of ISP-I in vivo using a tumour xenograft model.
Results
Our results showed that ISP-I significantly suppressed the growth of OS cells both in vitro and in vivo. Furthermore, ISP-I dose-dependently inhibited cell migration and invasion, and induced apoptosis and cell cycle arrest in OS cells. Mechanistically, ISP-I directly bound to TOP1 and inhibited DNA replication. Additionally, ISP-I significantly downregulated the ATR/CHEK1 pathway, which led to the suppression of DNA damage repair, ultimately augmenting DNA damage and triggering cell death.
Conclusions
In conclusion, our study suggests that ISP-I could be a novel TOP1 inhibitor that does not activate the ATR/CHEK1 DNA damage repair pathway. This characteristic allows ISP-I to synergistically inhibit OS cell proliferation, migration and invasion. ISP-I may represent a promising candidate for the treatment of OS.
1 INTRODUCTION
Osteosarcoma (OS) is a primary malignant bone tumour that mainly affects children and adolescents, originating from mesenchymal stem cells.1 While surgical and chemotherapeutic approaches have improved patient prognosis in recent decades, the problem of chemoresistance remains a significant challenge, particularly in patients with metastatic OS.2, 3 Therefore, there is a pressing need to develop novel anti-cancer agents for OS.
DNA topoisomerase catalyzes DNA strand breaks and binding and is therefore essential for DNA replication. Rapid DNA replication is a hallmark of malignancy, and blocking DNA replication can lead to mutations and/or chromosome rearrangements, ultimately triggering cell death.4 Thus, DNA topoisomerase inhibitors are potential anticancer agents because they help maintain strand breaks generated during replication by topoisomerases.5 In fact, anticancer agents targeting topoisomerase I (TOP1) and II (TOP2) have been widely used in cancer treatment.6-9 For OS, the TOP2 inhibitor adriamycin (ADM) is one of the most effective chemotherapeutic agents.10 In contrast, conventional TOP1 inhibitors such as irinotecan, topotecan, and camptothecin, have shown limited efficacy in OS and have rarely been used clinically11, 12 despite frequent TOP1 gene alterations in OS.13
The mechanisms underlying the insensitivity or resistance of cancers to conventional TOP1 inhibitors remain elusive. Previous studies have suggested that intracellular DNA damage response (DDR) signalling pathways are activated when TOP1 inhibitors affect DNA replication and cause DNA damage in cancer cells.14, 15 Among these pathways, it is well established that activation of ataxia telangiectasia and Rad3-related protein (ATR) contributes to the repair of DNA damage created by TOP1 inhibitors.16 ATR and its downstream checkpoint kinase 1 (CHEK1) compromise the cytotoxicity of TOP1 inhibitors by transiently arresting replication forks, limiting collisions between replication forks and TOP1 cleavage complexes and allowing the repair of broken replication forks.17, 18 Hence, activation of the ATR/CHEK1 pathway plays a critical role in drug resistance to TOP1 inhibitors. On the other hand, combined administration of ATR and/or CHEK1 inhibitors with TOP1 inhibitors has proven to be an ideal strategy to overcome drug resistance and enhance tumour-killing effects.17-19
In this study, we demonstrate that isovalerylspiramycin I (ISP-I), the main active component of a novel macrolide antibiotic carrimycin approved by the China National Medical Products Administration,20 exhibited significant antitumor activity in OS in vitro and in vivo. Mechanistically, TOP1 was identified and confirmed to be the direct target of ISP-I in OS. In addition to the inhibition of TOP1, ISP-I also significantly suppressed the ATR/CHEK1 pathway to inhibit DNA damage repair in OS cells. To our knowledge, ISP-I could be the first TOP1 inhibitor with ATR/CHEK1 inhibitory effects. Therefore, ISP-I is a promising new TOP1 inhibitor for use in OS treatment.
2 MATERIALS AND METHODS
2.1 Cell culture
From the Chinese Academy of Sciences & apos; Cell Bank of Type Culture Collection in Shanghai, China, 143B and Saos-2 human OS cell lines were obtained and cultured in Dulbecco's modified Eagle medium (DMEM) (Gibco, USA) supplemented with 10% fetal bovine serum (FBS, #10270-106, Gibco) and 1% penicillin-streptomycin in humidified air at 37°C and 5% CO2. Cells were regularly tested for bacterial contamination or mycoplasma infection. ISP-I (95.9% purity, Shanghai Tonglian Pharmaceutical Co., Ltd., Shanghai, China) was dissolved to 20 mM in dimethyl sulfoxide (DMSO, #D4540, Sigma‒Aldrich, St. Louis, MO, USA) and stored at –80°C.
2.2 Measurement of cell proliferation with CCK-8 assays
After digesting 143B and Saos-2 cells at the logarithmic growth stage in the supernatant culture medium with trypsin, 100 µl was inoculated into 96-well plates with a density of 104 cells/well and incubated at 37°C for 12 h. Different concentrations of ISP-I were added after 12 h, and CCK-8 (#96992, Dojindo Molecular Technologies, Dojindo, Japan) was added after 24 h. After one h, the absorbance value at OD450 was measured by an enzyme marker, the cell proliferation curve was plotted according to the absorbance value, and the IC50 was calculated.
2.3 Wound-healing assay
After inoculating the cells in a 6-well plate, 80% of them were scribed vertically with a 10 µl gun, then washed three times with PBS, incubated with varying concentrations of ISP-I, and removed after 24 h for observation. Using a microscope (10× objective, 1×71 Olympus), images were taken to ascertain the breadth of the wound.
2.4 Transwell invasion assay
143B and Saos-2 cells were treated with different concentrations of ISP-I for 24 h. Serum-free suspensions were prepared at 5.0×105, 200 µl of each well was inoculated in the upper chamber (Corning Inc., Corning, NY, USA), and 600 µl of culture medium containing 10% FBS was added to the lower chamber. Gently swabbing off the uppermost layer of cells, methanol was fixed for 20 min, then crystal violet was used for 20 min, PBS was washed thrice, and the cells were air-dried before inverted microscopy was employed to observe their migration.
2.5 Hematoxylin and eosin staining and immunohistochemical staining
Staining of hematoxylin and eosin (H&E) and immunohistochemical substances was done in accordance with the protocol outlined by Zhang et al.21 The antibody dilution ratio for TOP1 was 1:200
2.6 5-ethynyl-2′-deoxyuridine labelling and immunofluorescence
The cells were inoculated into 96-well plates. When the cell density reached 60%, different concentrations of ISP-I were added and cultured in the incubator for 24 h for EdU labelling and immunofluorescence (Guangzhou Ribo Biotechnology). The whole process can be divided into four stages: 5-ethynyl-2′-deoxyuridine (EDU) labelling, cell immobilization, Apollo staining and DNA staining. The following is a brief description of a well as an example: add 100 µl 50 µM EDU medium and incubate for 2 h, clean twice with PBS, then incubate with 4% paraformaldehyde at room temperature for 30 min, add 50 µl 2 mg/ml glycine solution to each well and incubate for 5 min, then add 100 µl 1X Apollo staining reaction solution and incubate at room temperature for 30 min, away from light. At long last, 100 µl of 1X Hoechst33342 reaction solution was added and left to incubate at room temperature and away from illumination for 30 min. After washing with PBS, observations could be made.
2.7 Cell apoptosis analysis
OS cells were inoculated in 6-well plates, and a culture medium containing different concentrations of drugs was added when the cell density reached 60%. Twenty-four hours later, all cells, including floating and adherent cells, were collected and subjected to Annexin V-isothiocyanate fluorescein and propidium iodide (PI) according to the manufacturer's protocol (#556547, BD Biosciences Pharmingen, CA, USA San Diego, CA) Apoptosis Detection Kit staining. The resulting fluorescence was assessed with fluorescence-activated cell sorting scan (FACS) flow cytometry (Becton Dickinson, Mountain View, CA, USA).
2.8 Cell cycle analysis
The OS cells, after having been exposed to varying concentrations of ISP-I for 24 h, were harvested and fixed in 70% ethanol overnight at –20°C before being stained with PI. FACS flow cytometry (Becton Dickinson) was employed to detect the DNA content, as per the manufacturer's instructions. The cell cycle distribution was determined using FlowJo software 8.7.1 (Tree Star Inc., Ashland, OR, USA).
2.9 Identification of ISP-I target proteins in OS
To explore the direct cell target of ISP-I, we applied a surface plasmon resonance-high-performance liquid chromatography-mass spectrometry (SPR-HPLC‒MS) assay as described previously.22 Briefly, ISP-I was immobilized on the surface of the chip, and 143B and Saos-2 cell lysates were used as the liquid phase. Using a photo-crosslinked SPR sensor chip to detect the compound-protein interaction, rapamycin and biotin were selected as systematic controls. To monitor the enrichment process of the target protein, we performed a real-time SPR experiment using the bScreen LB 991 Label-free Microarray System (BERTHOLD TECHNOLOGIES, Germany). Subsequently, an LC-MS experiment was used to identify the types and relative abundance of proteins captured on the surface of the chip.
2.10 Molecular docking
The molecular docking assays between ISP-1 and TOP1 were generated using the Protein Preparation Wizard module of Schr€odinger Maestro 9.3 software as described previously.22 Briefly, precision was set as extra precision, ligand sampling was set as flexible, and other parameters were set at the default values. Subsequently, the residual compounds were subjected to visual analysis.
2.11 Direct enzyme activity assay
Samples containing different concentrations of ISP-I were prepared, and each sample contained 1 µg of pbR322 DNA (#3050, Takara, Japan), 5 U of TOP1 (#2240A, Takara, Japan), different concentrations of ISP-I and enzyme-free water. The samples were reacted at 37°C for 15 min. At the end of the reaction, electrophoresis was performed using a 1.2% agarose gel at 80 V for 60 min, and images were captured using a gel imaging system.
2.12 Intracellular enzyme activity assay
Cells treated with different concentrations of ISP-I were collected, nuclear proteins were extracted, and the protein concentration of each sample was made consistent using the BCA protein quantification method. The extracted proteins were incubated with 1 µg pbR322 DNA and 5 U TOP1 at 37°C for 15 min and then subjected to agarose gel electrophoresis, and images were collected.
2.13 The thermal shift assay
The thermal shift assay was performed as previously described.23 For the living cell temperature-dependent thermal shift assay, 143B and Saos-2 cells were incubated with 10 µmol/L and 20 µmol/L ISP-I for 18 h, and then the cells were heated at each temperature point from 35 to 60°C for 3 min. The samples were centrifuged at 20 000 × g for 10 min at 4°C to separate the supernatants and pellets and then separated using 10% sodium dodecyl-sulfate polyacrylamide gel electrophoresis for immunoblotting analysis of TOP1.
2.14 Western blotting
The expression of proteins in OS cells was quantified using a BCA Protein Assay Reagent Kit (#23228, Thermo Fisher Scientific) as described previously.24 Rabbit polyclonal cleaved caspase-3 (#9664), BCL-2 (#3498), BAX (#5023), cyclin D1 (#55506) and CDK4 (#12790) antibodies were purchased from Cell Signaling Technology (Boston, USA). The TOP1 (#ab121681), ATR (#ab2905), CHEK1 (#ab32531) and p-CHEK1 (#ab278717) antibodies were purchased from Abcam (USA), and the band density was quantified using ImageJ software 1.8.0.
2.15 Detection of γH2AX by immunofluorescence
Logarithmic growth stage OS cells were inoculated on 6-well plates and treated with different concentrations of drugs for 24 h. The cells were fixed with 4% paraformaldehyde for 30 min, phosphate-buffered saline (PBS), 0.3% Triton-X 100 permeable cells for 30 min, and PBS washing; 5% BSA sealing solution for 1 h; rabbit anti-γH2Ax antibody (1∶100) (#81299, Abcam, USA) was added overnight at 4°C. After washing with PBS, a fluorescent secondary antibody against rabbit (1∶200) (#8878, CST, USA) was added at room temperature for 1 h, and the cells were washed with PBS. Then, 4′,6-diamidino-2-phenylindole solution was added and incubated for 10 min away from light. After washing with PBS, an appropriate amount of anti-fluorescence quenching agent was added, and the images were observed using fluorescence microscopy.
2.16 Construction of plasmids and transfection
The TOP1 overexpression pcDNA3.1 plasmid was constructed by Foresight Biotechnology Co., Ltd. (Shanghai, China). The plasmid was transfected into OS cells using Lipofectamine 3000 (#L3000015, Invitrogen) following the manufacturer's instructions.
2.17 Quantitative real time polymerase chain reaction
RNA was extracted from 143B and Saos-2 cells using the TRIzol kit, and cDNA was synthesized according to the reverse transcription kit instructions. A qPCR kit was used for amplification reactions. Using β-actin as the internal reference, the upstream primer: 5′-CCCTGGAGAAGAGCTACGAG-3′, the downstream primer: 5- GGAAGGAAGGCTGGAAGAGT-3′; TOP1 upstream primer: 5′- TCGAAGCGGATTTCCGATTGA −3′, downstream primer: 5′- CTTTGTGCCGGTGTTCTCGAT −3′. The mRNA expression was calculated, and the experiment was repeated three times.
2.18 Untargeted metabolomic relative quantitative analysis
To screen for differential metabolites in 143B and Saos-2 cells after ISP-I treatment, untargeted metabolomics profiling was performed using ultra-HPLC (1290 Infinity LC; Agilent Technologies, USA) coupled with a quadrupole time-of-flight system (AB Sciex TripleTOF 6600; AB SCIEX, USA) at Shanghai Applied Protein Technology Co., Ltd. as described previously.24 MS was executed with an electrospray ionization (ESI) source running in both positive (ESI+) and negative (ESI-) modes. After normalization to the total peak intensity, the data were exported for bioinformatics analysis.
2.19 Mouse xenograft experiment
Female M-NSG (purchased from Diocesan Pharmachem, China) were used at 4–6 weeks of age, with a body mass of approximately 20 g. 143B cells, logarithmically grown, were digested with trypsin and washed twice with PBS. Subcutaneous tissue of the mice's right hind leg was then injected with 2×106 cells. Randomly dividing the animals into two groups, when the tumours had grown to around 100 mm3, the DMSO group was given a blank control and the ISP-I group a treatment dose of 60 mg/kg at 1-day intervals. Mouse body weight and tumor length and width were measured at 5-day intervals, and tumor volume was calculated using the formula (length×width2)/2. The mice were sacrificed 30 days after treatment began and the tumors were removed.
2.20 Statistics and bioinformatics analysis
Continuous variables are presented as the mean ± SD. GraphPad Prism 7 (GraphPad Software Inc., CA, USA) was employed to conduct data analysis. Statistically significant was the P value of less than 0.05. For metabolomics data, differentially abundant metabolites were analyzed using an unpaired Student's t-test. Variable importance in the projection (VIP) value > 1 and p < 0.05 were considered statistically significant. The above-obtained metabolites were subsequently subjected to KEGG enrichment analysis.
3 RESULTS
3.1 ISP-I inhibited the proliferation, migration and invasion of OS cells in vitro
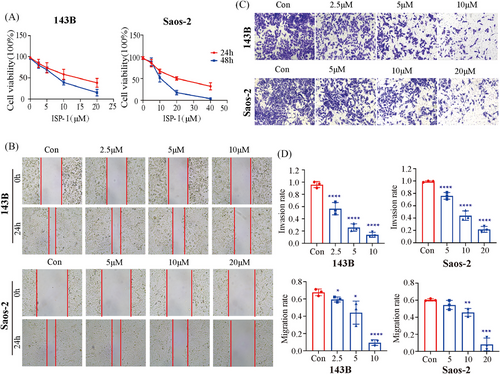
To explore the inhibitory effect of ISP-I on OS cells in vitro, CCK-8, wound healing and Matrigel Transwell invasion assays were performed on 143B and Saos-2 cells. The CCK-8 assay showed that ISP-I significantly inhibited cell proliferation in a time- and concentration-dependent manner, with IC50 values of 8.7 and 17.8 µmol/L at 24 h for 143B and Saos-2 cells, respectively (Figure 1A). The wound healing assay and Matrigel Transwell invasion assay showed that ISP-I dose-dependently inhibited OS cell migration (Figure 1B,D) and invasion (Figure 1C,D).
3.2 ISP-I inhibited the growth of subcutaneously transplanted tumours in vivo
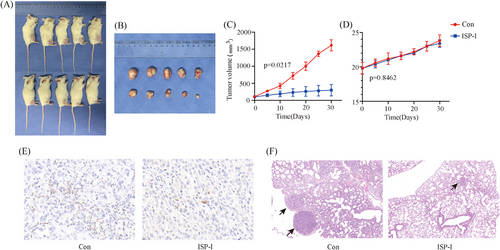
To evaluate the inhibitory effect of ISP-I in vivo, we conducted xenograft mouse experiments. Mice were sacrificed, and tumours were harvested after 30 days of treatment with ISP-I (Figure 2A,B). As a result, ISP-I significantly inhibited the increase in tumour volume in 143B xenografts (Figure 2C), Moreover, there was no significant difference in body weight between the two groups of mice (Figure 2D), suggesting that ISP-I was well tolerated. Immunohistochemical staining of mouse tumour tissues revealed a reduction in TOP1 expression in the ISP-I treated group, consistent with our expectations (Figure 2E). Lung H&E staining results indicated that ISP-I significantly inhibited the lung metastasis of OS cells (Figure 2F).
3.3 ISP-I inhibited DNA replication in OS cells
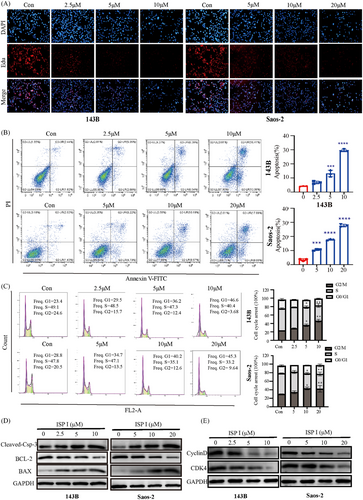
To evaluate the effect of ISP-I on DNA replication, we performed EdU labelling and immunofluorescence assays. The results showed that the number of EdU-labeled cells decreased in a dose-dependent manner following ISP-I treatment (Figure 3A).
3.4 ISP-I induced apoptosis and cell cycle arrest in OS cells
Apoptosis analysis revealed that ISP-I induced dose-dependent apoptosis in 143B and Saos-2 cells (Figure 3B). This was supported by the downregulation of BCL-2 protein and upregulation of cleaved-Caspase-3 and BAX protein using western blot assay (Figure 3D). Flow cytometry was used to analyze the effects of different concentrations of ISP-I on the cell cycle of OS cells (Figure 3C). The results showed that compared with the control group, the number of cells in the G1 phase was significantly increased in the ISP-I experimental group, and this effect was concentration-dependent. Western blot analysis demonstrated a significant reduction in the expression levels of CDK4 and Cyclin D1 in the G1 phase of OS cells following ISP-I treatment (Figure 3E).
3.5 ISP-I directly bound to TOP1 and inhibited its activity
To investigate the direct targets of ISP-I in OS cells, we conducted a target screening assay using SPR-HPLC‒MS. The assay involved immobilizing ISP-I on a chip, incubating the chip with 143B and Saos-2 cell lysates, and identifying the possible targets of ISP-I. A total of 24 proteins from both 143B and Saos-2 cells were identified (Figure 4B,C). Among these proteins, TOP1 drew our attention due to its critical role in DNA replication and apoptosis.25 The molecular docking analysis revealed that ISP-I binds with Arg-488 and Arg-590 residues in the conserved core domain of TOP1 (Figure 4D).26 To confirm whether ISP-I directly binds to TOP1, thermoshift assays were performed. Our results indicated that TOP1 in the ISP-I-treated group exhibited increased thermal stability and stronger bands compared to TOP1 in the control group as the temperature increased, supporting the direct binding of ISP-I to TOP1 (Figure 4E). In line with the abovementioned data, western blot assays showed that the protein expression of TOP1 in OS cells was downregulated dose-dependently by ISP-I.
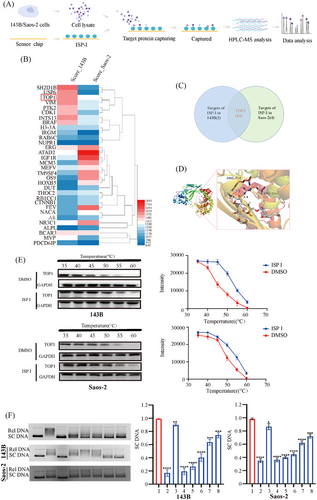
To further validate these findings, we performed extracellular and intracellular TOP1 enzyme activity assays (Figure 4F). The results of agarose gel electrophoresis showed that the addition of TOP1 deconvoluted pBR322 DNA into relaxed DNA (Lane 2). As a positive control, CPT can inhibit this deconvolution effect (Lane 3). Similarly,
The lanes containing ISP-I showed an increase in superhelical DNA with increasing drug concentration (Lanes 5–8), suggesting that ISP-I had an inhibitory effect on TOP 1 enzyme activity.
3.6 ISP-I triggered DNA damage
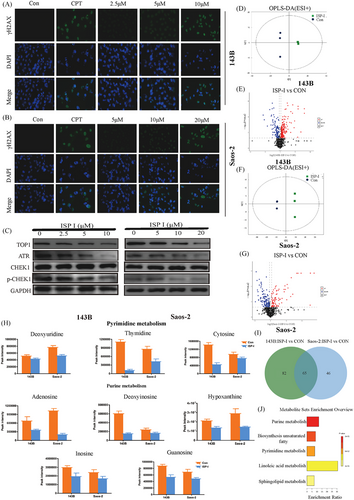
Since inhibition of TOP1 can cause DNA damage, while suppressing the ATR/CHEK1 pathway impedes DNA damage repair, we assessed whether ISP-I treatment augmented DNA damage in OS cells. As expected, immunofluorescence staining analysis revealed that ISP-I-treated cells showed higher quantities of γH2AX, a sensitive marker of DNA damage (Figure 5A,B).
3.7 ISP-I inhibited the ATR/CHEK1 pathway
As mentioned previously, ISP-I is directly targeted to TOP1 to exert inhibitory effects on OS. We further investigated whether the ATR/CHEK1 pathway, which is often activated in response to DNA damage caused by TOP1 inhibitors, was affected by ISP-I treatment.16 Notably, after exposure to different concentrations of ISP-I, the ATR/CHEK1 pathway in OS cells was suppressed dose-dependently instead of being activated. The protein expression of ATR and p-CHEK1 decreased in a dose-dependent manner, while the protein expression of CHEK1 remained relatively unchanged (Figure 5C).
3.8 ISP-I treatment suppresses pyrimidine and pyrimidine metabolism
Given the observed effects of ISP-I on the inhibition of DNA replication and suppression of DNA damage repair, we performed untargeted metabolomics using high resolution, high throughput, and highly sensitive technology to detect changes in metabolite profiles after ISP-I treatment. The results showed that 66 and 49 differentially abundant metabolites were detected in ESI+ mode in 143B and Saos-2 cells, respectively (Figure 5D–G), while 81 and 62 metabolites were detected for the same comparisons in ESI- mode (Figure S1). Then, we combined the significantly expressed metabolites in 143B and Saos-2 cells and found that 65 metabolites were common (Figure 5I and Table S1). Enrichment analysis revealed that the top five differentially regulated pathways after ISP-I treatment were purine metabolism, biosynthesis of unsaturated fatty acids, pyrimidine metabolism, linoleic acid metabolism and sphingolipid metabolism (Figure 5H). Importantly, the key intermediate metabolites of pyrimidine metabolism and pyrimidine metabolism pathways were significantly downregulated, which further validated that ISP-I inhibited DNA replication and caused DNA damage in OS cells by targeting TOP1 and suppressing the ATR/CHEK1 pathway (Figure 5J).
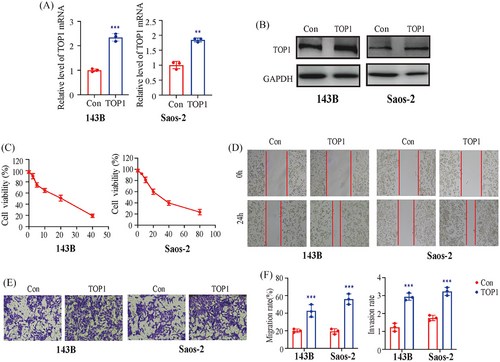
3.9 Overexpression of TOP1 reversed the inhibitory effect of ISP-I
To verify the role of TOP1 in the effects of ISP-I on OS cells, we transfected OS cells with a TOP1 overexpression plasmid (Figure 6A,B). As expected, the overexpression of TOP1 significantly increased the viability of 143B and Saos-2 cells, and the IC50 of ISP-I for 143B and Saos-2 cells at 24 h increased to 19.2 and 30.6 µM, respectively (Figure 6C), which was significantly higher than that in the null group. Similarly, the wound healing assay and Transwell assay revealed that overexpression of TOP1 attenuated the inhibitory effect of ISP-I on the migration and invasion of OS cells (Figure 6D–F). These data confirmed that ISP-I suppresses OS cells by targeting TOP1.
4 DISCUSSION
Repurposing old drugs has become an attractive approach to drug discovery due to its potential for cost and time savings. Studies have shown that macrolide antibiotics, such as rapamycin, clarithromycin, and azithromycin, possess anticancer properties and could be candidates for cancer treatment.27, 28 As a novel macrolide antibiotic, ISP-I also has been found to have potent antitumor effects in many malignancies.20, 29, 30 The antitumor mechanisms of ISP-I differ greatly in different cancers. For example, ISP-I can reduce the expression of VEGF and PD-L1 in hepatocellular carcinoma cells.29 ISP-I targets SELH and induces the accumulation of ROS and cancer cell-specific genomic instability in glioblastoma,30 and causes ROS accumulation and inhibits the PI3K/AKT signalling pathway In non-small cell lung cancer.20 Notably, ISP-I was proposed as a promising antitumor agent due to its unique multitarget ability, which may decrease the probability of chemotherapy resistance.20 However, the effects of ISP-I on OS and the underlying mechanisms remain unexplored.
In this study, we found that ISP-I dose-dependently inhibited cell proliferation, migration, invasion and induced apoptosis and cell cycle arrest in 143B and Saos-2 OS cells. To explore the direct cell target of ISP-I, we designed an SPR-HPLC‒MS assay and found that it selectively targets TOP1. We validated these findings using a thermal shift assay, which demonstrated that ISP-I significantly increased the thermal stability of TOP1, indicating a direct interaction between ISP-I and TOP1. Subsequent assays of TOP1 enzyme activity showed that ISP-I inhibited TOP1 enzyme activity both extracellularly and intracellularly, leading to an increase in supercoiled DNA in a concentration-dependent manner. Accordingly, ISP-I dose-dependently downregulated the protein expression of TOP1 in OS cells. To confirm the key role of TOP1, we performed a reverse test and found that the suppression of OS cell proliferation, migration, and invasion by ISP-I was significantly reduced after overexpressing TOP1.
Given that inhibition of TOP1 can suppress DNA replication,5 we assessed DNA replication in ISP-I-treated OS cells using EdU labelling. As a result, ISP-I reduced EdU incorporation into DNA in a dose-dependent manner. These findings revealed that ISP-I inhibited DNA replication and further supported that ISP-I targets TOP1 to inhibit OS.
Typically, when TOP1 inhibitors affect DNA replication and cause DNA damage in cancer cells, DDR pathways are then activated to maintain the integrity and stability of the cellular genome.14, 15 To repair TOP1 poison-induced DNA lesions, activation of ATR signalling is required due to the runoff of the replication fork and the presence of long single-stranded DNA.16 ATR can phosphorylate CHEK1 to harness cell cycle arrest. In the present study, however, the protein expression of ATR and p-CHEK1 in OS cells was significantly downregulated after ISP-I treatment, while the protein expression of CHEK1 remained unaffected. This suggested that ISP-I inhibited TOP1 and caused DNA damage without activation but suppression of the ATR/CHEK1 pathway, thereby achieving tumour cell killing. In particular, the percentage of cells in the S-phase markedly decreased according to cell cycle analysis, similar to a previous study.31 To further validate these findings, we assessed whether ISP-I treatment augmented DNA damage in OS cells. As expected, γH2AX staining analysis revealed that ISP-I-treated cells showed higher amounts of DNA damage.
Cancer cells with increased DNA repair activities may be less sensitive to TOP1 inhibitors, which partially explains the resistance of some malignancies to this class of drugs.11 For OS, Li et al. showed that ATR is overexpressed in human OS tissues, and ATR regulates DNA damage repair and promotes OS cell growth, proliferation and migration through a CHEK1-dependent pathway, while the ATR selective inhibitor Berzosertib leads to characteristic cytoplasmic vacuolization and OS cell death.32 These results provide a rationale to combine the administration of TOP1 inhibitors with inhibitors of DNA damage repair pathways to overcome drug resistance and enhance antitumor activity.17-19 From this perspective, ISP-I might be superior to conventional TOP1 inhibitors because of the synchronous blocking of both TOP1 and the ATR/CHEK1 DNA damage repair pathway in OS. Moreover, previous preclinical and clinical studies suggested that there was no significant tissue toxicity after ISP-I treatment.20, 33 Taken together, ISP-I could emerge as a potential therapeutic strategy in OS.
In this study, we demonstrated that ISP-I directly binds to and inhibits TOP1, leading to DNA damage. Notably, ISP-I also inhibits the ATR/CHEK1 pathway, preventing DNA damage repair and increasing the number of OS cell deaths to some extent. In summary, ISP-I may be a novel TOP1 inhibitor and a promising candidate for antitumor therapy.
AUTHOR CONTRIBUTIONS
Yan Zhou and Jianjun Zhang designed research; Jinrong Liang, Peng Zhang and Kunyan He performed research; Yawen Zhang, Xiaomin Ding, Qian Li and Wang Zhou performed data analysis and prepared figures; Jinrong Liang and Jianjun Zhang wrote the paper.
ACKNOWLEDGEMENTS
This work was supported by grants from the National Natural Science Foundation of China (82274261 and 82102866), Science Foundation of Shanghai Sixth People's Hospital(No. ynqn202114) and Shanghai Pujiang Program (21PJD051).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Animal experiments are approved by the Ethics Committee of Shanghai Sixth People's Hospital and are conducted in the animal room of Shanghai Sixth People's Hospital in accordance with the following Guidelines on the Care and Use of Experimental Animals.




