Dead but functional liquid nitrogen-treated umbilical cord mesenchymal stem cells accelerate full-thickness skin wound healing
[Correction added on 30 November 2023; after first online publication missing sub-sections have been added.]
Yan Yang, Yuqing Feng and Meirong Hu contributed equally.
Funding information:
National Natural Science Foundation of China, Grant Nos.: 81871150, 32170865, 82071634; Natural Science Foundation of Guangdong Province, Grant No: 2022A1515012178; the Science and Technology Plan Project of Guangzhou, Grant No.: 202002030168; the Science and Technology Plan Project of Guangzhou, Grant No.: 202103030003; Guangdong Key Areas R&D Program, Grant No.: 2022B1111080007
Abstract
Background
The direct application of umbilical cord-derived mesenchymal stem cells (UCMSCs) for promoting skin wound healing and regeneration is challenging due to strict maintenance requirements and unpredictable differentiation results.
Methods
We developed dead but functional liquid nitrogen-treated UCMSCs (LNT-MSCs) by rapidly immersing live UCMSCs (live-MSCs) in liquid nitrogen.
Results
The LNT-MSCs maintained similar cellular structures and surface markers to those of live-MSCs. We evaluated the therapeutic effects of both live-MSCs and LNT-MSCs on full-thickness skin wound healing in rats. Our results showed that the LNT-MSCs accelerated wound closure by enhancing the proliferation and migration of skin cells, promoting angiogenesis, and inducing a favourable macrophage phenotype shift. The regenerative healing effect of LNT-MSCs was comparable to that of live-MSCs, making them a potential alternative strategy for accelerating wound closure that avoids unpredictable differentiation results and creates a ready-to-use cell bank for clinical applications.
1 INTRODUCTION
The skin protects subcutaneous tissue and maintains the body's physiological balance.1, 2 The skin is highly vulnerable to infections resulting from injuries that compromise its integrity and function.3 Healing cutaneous wounds involve four interrelated stages: hemostasis, inflammation, proliferation and remodelling. In most cases, skin defects can be effectively regenerated within 7– 14 days. Serious skin defects that extend to the skeleton heal with difficulties and usually form non-functional scar tissues.4, 5 Therefore, when there is a critical size (> 10 mm in diameter) injury to the skin, it is important to accelerate wound healing.6
In the last decade, considerable effort has been devoted to identifying treatments that improve wound healing. Mesenchymal stem cells (MSCs) promote tissue regeneration and wound healing.7-9 Various sources of MSCs exist, including bone marrow, adipose tissue and umbilical cord blood.10 Owing to their multilineage differentiation and self-renewal properties, MSCs accelerate cutaneous wound healing by promoting angiogenesis and modulating the local inflammatory microenvironment.11 Compared with adult-derived MSCs, human umbilical cord-derived MSCs (UCMSCs) are easier to obtain and have a greater expansion capacity, and lower immunogenicity.12 UCMSCs have been used to accelerate diabetic wounds closure.13 The UCMSCs promote skin healing, improve the function of fibroblasts and endothelial cells and regulate gene expression.14 In type II diabetic mice, UCMSCs induce macrophage polarisation to alleviate islet dysfunction, thereby attenuating skin wound inflammation and promoting angiogenesis.15 Since UCMSCs could secrete growth factors, cytokines and exosomes, they can target inflammatory tissues, regulate excessive immunity and inflammation, and promote tissue repair.16 Despite this, MSCs have many limitations when applied directly to tissue injuries. With cell culture expansion, the risk of teratoma occurrence increases.17, 18 In addition, the extraction, transportation, and expansion of MSCs are invasive, time-consuming, and difficult to perform clinically.19 Therefore, there has been increasing interest in the use of lyophilisation of MSCs in regenerative medicine and cell-based therapies. Lyophilisation is a process of freeze-drying the secretome of MSCs into a powder form that can have beneficial effects on wound healing, bone regeneration and fat grafting.20-22 It has been reported that lyophilised UCMSCs powder can also accelerate the wound closure.21 However, MSC lyophilised powder may also have some drawbacks, such as rapid clearance and short half-life due to exposure of soluble factors to tissues.22
Over the past few years, there have been several studies indicating that extracellular vesicles (EVs) and apoptotic bodies (ABs) derived from MSCs play a significant role in wound healing.23-27 However, critical challenges include the standardisation of the isolation and purification and the optimisation of methods for creating quality EVs and ABs’ populations.19, 28, 29 Recently, a study investigated the effects of cryo-shock treatment on acute myeloid leukaemia (AML) cells and found that cryo-shocked AML cells maintain their bone marrow homing capability.30 We thus hypothesised whether the function of live MSCs can be attributed to their intracellular proteins and cytoskeletal structure.
In this study, we obtained dead but still functional liquid nitrogen-treated UCMSCs (LNT-MSCs) using a liquid nitrogen-based cryo-shocking process. We evaluated the effects of both live live-MSCs and LNT-MSCs on skin wound healing. And our findings suggested that LNT-MSCs have the potential to provide resemble therapeutic benefits to live-MSCs. The LNT-MSCs may represent a novel clinical strategy for accelerating wound closure.
2 MATERIALS AND METHODS
2.1 Animal and cells
We purchased Sprague–Dawley (SD) rats (160 20 g) from the Experimental Animal Center of Guangdong Province, China (certificate No. SYXK (Guangdong) 2018−0002).
2.2 Preparation of liquid nitrogen-treated UCMSCs
Live-MSCs grown to 80% fusion and digested it. Then, the cells were resuspended in an uncontrolled-rate cryopreservation medium (Cyagen, NCRC-10001-50), and placed in a liquid nitrogen tank for 72 h.
2.3 Cellular sizes
Cells were suspended in phosphate-buffered saline (PBS) (pH 7.4). And the cellular size was measured using Nano Measurer software.
2.4 Cellular structure
To determine the cellular structure of live-MSCs and LNT-MSCs, fluorescein isothiocyanate (FITC)-phalloidin (Beyotime, Beijing, China) was used to stain the cells. After being fixed with 4% paraformaldehyde (PFA), blocked with immunostaining blocking solution, and incubated with FITC-phalloidin overnight at 4°C. The 4′,6-diamidino-2-phenylindole (DAPI) was used to stain nucleus. Green FITC-phalloidin staining indicated the cellular structure, and blue DAPI staining indicated the nuclei.
2.5 Cell viability
Calcein-AM/PI was used to determine live-MSCs and LNT-MSCs viability, following the manufacturer's instructions (Beyotime Institute of Biotechnology). Photographs were taken using an inverted confocal laser microscope, and measurements were made using ImageJ software.
2.6 Flow cytometric analysis of cell phenotype
To identify the expression of MSC surface markers, LNT-MSCs and live-MSCs were incubated with fluorescein FITC-conjugated rabbit anti-CD29 (ThermoFisher Scientific, PA5-85943) and anti-CD44 (ThermoFisher Scientific, PA5-29591) antibodies. These cells were subsequently analysed through flow cytometry using CytoFLEX (Beckman Coulter, Shanghai, China).
2.7 Scanning electron microscopy
The cells were fixed in 3.5% glutaraldehyde (G1102, Servicebio, Wuhan, China) for 4 h. After washing with 0.1 M PBS, these cells were fixed for 1 h with 1% osmium tetroxide (75632, Sigma, USA). Then, the cells were dehydrated with graded ethanol. Scanning electron microscopy (SEM) analysis was performed after coating the silicon with a thin layer of gold (HITACHI Regulus 8100).
2.8 Skin wound healing models
Rats were randomly divided into three groups of seven and their back hair was removed. On the day of modelling, the rats were fasted for 12 h and then anaesthetised with 2% pentobarbital sodium by intraperitoneal injection. On the dorsal surface, a 1-cm-diameter wound was created. Live-MSCs and LNT-MSCs were resuspended in PBS (1 × 107 cells/mL) and directly added to the wound (100 μL/cm2).The control group received PBS. The wound was then covered with surgical dressing. After surgery, wound areas were measured on days 0−21, and wound healing rates were calculated with ImageJ.
2.9 Analysis of wound closure rate
Complete wound healing refers to the time from the day of administration to the complete disappearance of the wound. On the 3rd, 7th, 14th and 21st days after surgery, calculations of wound healing rate were conducted using ImageJ software. The healing rate was calculated as follows:
Wound healing rate (%) = [(area of original wound − area of unhealed wound)/area of original wound]× 100%.
2.10 Histological analysis
Tissue from full-thickness traumatic wounds was harvested and fixed with PFA. Hematoxylin and eosin staining (H&E), and Masson's trichrome staining were performed by Wuhan Servicebio Technology (Wuhan, China). Images were captured using a Nikon DS RI orthographic microscope.
2.11 Analysis of epidermal thickness
Following staining, we randomly selected eight fields from each animal sample and captured images at a magnification of 200×. Epidermal thickness was measured using ImageJ by selecting at least five different fields in each image, took multiple measurements across the field to obtain a representative value. Then, the average thickness was calculated for each animal.
2.12 Immunofluorescence analysis
To prepare the samples for analysis, we used 0.05% Triton X-100 (Sigma–Aldrich, USA) to permeabilise both cells and tissue sections. Subsequently, we blocked the samples with 1% bovine serum albumin (BSA, Sigma–Aldrich, USA). After overnight incubation with the primary antibody, we incubated the samples at room temperature for 1 h with the fluorescence secondary antibody (Sigma–Aldrich, USA). DAPI staining (Sigma–Aldrich, USA) was then performed for 5 min at room temperature on the nuclei. Antibody information is listed in Table S1. Images were captured using a Nikon DS RI orthographic microscope. Then, the average fluorescence intensity (a.u.) was analysed using ImageJ software. The a.u. was automatically extracted and plotted using the ImageJ software. Then, the cytoplasmic fluorescence intensity/nuclear ratios were calculated by the formula [a.u. in cytoplasm]/[a.u. in nucleus]. The average fluorescence intensity for each condition was then calculated based on an analysis of ten images.
2.13 Cell migration assay
Transwell and cell scratch assays were used to determine cell migration. We seeded NIH/3T3, HaCaT and HUVEC cells in a plate and allowed them to culture for 24 h.For each well, a 200 mL pipette tip was used to make three parallel scratches. The width of each scratch was measured as the baseline value. Live-MSCs and LNT-MSCs were seeded in upper chamber of a Transwells (4-μm pore filters; Corning, USA), respectively. These Transwells were cultured with NIH/3T3, HaCaT or HUVEC cells for 24 h. Images were taken using ImageJ software and a light microscope (Leica®, Germany) to determine the scratch width. The degree of scratch healing can be calculated by the following formula: Wound closure (%) = ((scratch width(0 h) − scratch width(24 h))/scratch width(0 h)) × 100%.
2.14 Cell proliferation assay
To assess the impact on the growth of NIH/3T3 cells, HaCaT cells and HUVECs, we utilised a Transwell system with a pore size of 4 μm (Corning, USA). NIH/3T3 cells, HaCaT cells and HUVEC cells (1×105 cells/well) were seeded on the lower chamber of the Transwell system, and live-MSCs and LNT-MSCs were added to the upper chamber. The Cell Counting Kit-8 (CCK-8) (Sigma–Aldrich) was used to measure cell proliferation.
2.15 Western blot analysis
To separate the proteins, we used a 12% gradient SDS–PAGE and transferred them onto a polyvinylidene fluoride (PVDF) membrane (Millipore, Billerica, MA, USA). After overnight incubation with primary antibodies, secondary antibodies were incubated at room temperature for 1 h. The target proteins were then detected by applying an enhanced chemiluminescence solution. We normalised the protein expression levels to that of β-actin.
2.16 Anti-inflammatory capacity analysis of liquid nitrogen-treated UCMSCs
To assess the effect of LNT-MSCs on the conversion of macrophages from the M1 phenotype to the M2 phenotype, we seeded 1 × 105 RAW264.7 cells (from Cyagen, OriCellbio, USA) in 24-well plates. After 24 h of cell culture, we induced the M1 phenotype by treating the cells with lipopolysaccharide (LPS, 1 μg/mL) (purchased from Sigma–Aldrich, LPS, 297-473-0) for 24 h. We seeded live-MSCs or LNT-MSCs (1×105 cells) in the upper chamber of a Transwell system (Corning, USA) with 4-μm pore filters. The upper chamber were then placed into a 24-well plate and co-cultured with macrophages for 48 h. To distinguish between the M1 and M2 phenotypes of macrophages, we used cell markers CD68 (for M1 phenotype) and CD206 (for M2 phenotype). The expression of CD68 (ThermoFisher Scientific, MA5−13324) and CD206 (ThermoFisher Scientific, MA5-32498) in co-cultured macrophages was detected by immunofluorescence.
2.17 Ribonucleic acid (RNA) extraction and qPCR
- β-actin-F: 5′-AAGTCTTCGGACGCAAGAAA
- β-actin-R: 5′-TTGCCCAGAAGCAGAACAG
- IL-1β-F: 5′-AATCTCACAGCAGCATCTCGACAAG
- IL-1β-R: 5′-TCCACGGGCAAGACATAGGTAGC
- TGF-β1-F: 5′-GACCGCAACAACGCAATCTATGAC
- TGF-β1-R: 5′-CTGGCACTGCTTCCCGAATGTC
- TNF-α-F: 5′-ATGGGCTCCCTCTCATCAGTTCC
- TNF-α-R: 5′-CCTCCGCTTGGTGGTTTGCTAC
2.18 Statistical analysis
The reported results consist of either the mean ± standard deviation or the mean ± standard error of the mean. Two groups were compared using Student's t-tests, while three or more groups were compared using ordinary one-way analysis of variances. GraphPad Prism software was utilised for all statistical analyses. Full data for quantification of images was listed in Table S2. Statistical significance was established as indicated by asterisks *p < .05, **p < .01, ***p < .001 and ****p < .0001.
3 RESULTS
3.1 Preparation and characterisation of cryo-shocked umbilical cord-derived mesenchymal stem cells
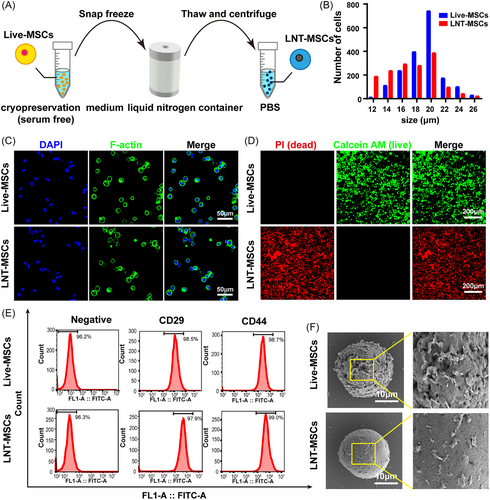
UMSCs were suspended in cryopreservation buffer and stored for 72 h in liquid nitrogen. The liquid nitrogen treated UCMSC (LNT-MSC) were then thawed at 37°C and washed with PBS (Figure 1A). Following exposure to liquid nitrogen, there was a reduction in cellular size observed, with LNT-MSCs exhibiting an average size of 18.47 μm and live-MSCs measuring at 20.04 μm on average (Figure 1B). The cellular structure of LNT-MSCs was found to be similar to that of live-MSCs (Figure 1C). Nearly, all LNT-MSCs exhibited positive PI staining (indicating dead cells) (Figure 1D). Additionally, cell counting kit-8 (CCK8) assay also showed that LNT-MSCs did not exhibit proliferative activity (Figure S1A). The MSC surface marker, CD29 and CD44, were expressed in LNT-MSCs, indicating that these cells retained MSC markers expression (Figure 1E). SEM images showed that both live-MSC and LNT–MSC exhibited a spherical shape with an intact membrane structure. Specifically, the SEM images revealed that the LNT-MSCs had smoother cell membrane compared to live-MSCs (Figure 1F, Figure S1B). These data suggested that UCMSCs treated with liquid nitrogen-based cryo-shocking retain their cellular morphology, membrane structure and surface markers despite being dead.
3.2 Liquid nitrogen-treated UCMSCs accelerated wound healing processes
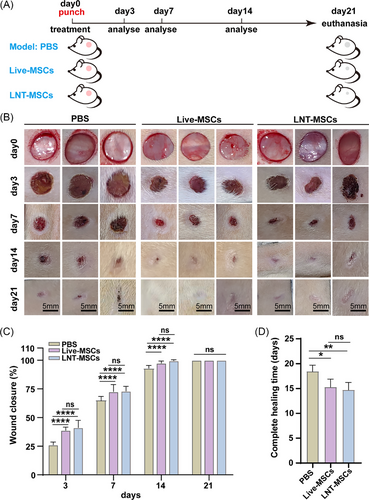
LNT-MSCs, live-MSCs and PBS were applied on the wounds. PBS was used as the negative control (Figure 2A). During the wound healing process, all mice displayed normal activity and appearance. After 3 days of treatment, the wounds in LNT-MSCs and live-MSCs treatment group showed certain degree of shrinkage. In comparison to PBS, wound healing rates in the LNT-MSCs and live-MSC groups significantly improved on days 3 and 7. The wounds in the LNT-MSCs and live-MSC groups healed completely on day 14 after surgery, indicating that the healing rate in these groups was faster than that in the PBS group. As expected, there were no significant differences in wound closure rate and healing time between the LNT-MSCs and live–MSCs treatment (Figure 2B–D and Figures S2 and S3).
On day 3, the epidermal structures in all groups exhibited an evident coloboma. PBS treatment group showed a high number of inflammatory cells, whereas LNT-MSCs and live-MSCs’ groups showed a lower number of inflammatory cells (Figure 3A). After 7 days of treatment, the granulation layer formation was observed in all wounds. We assessed the thickness of the epidermis in the closed region of the wounds on days 7 and 14. On day 7, the thickness of the epidermis was significantly increased in the LNT-MSC (201.60 μm) and live-MSC groups (200.00 μm) compared to the PBS group (174.71 μm). There was a greater thickness of epidermis in the LNT-MSCs treatment group than in the PBS treatment group, implying that treatment with LNT-MSCs treatment accelerated the re-epithelialisation. LNT-MSCs and live-MSCs groups had epidermis that was closer to normal skin thickness by day 14 (Figure 3B). And there was no significant in thickness of the epidermis between LNT-MSCs and live-MSC groups.
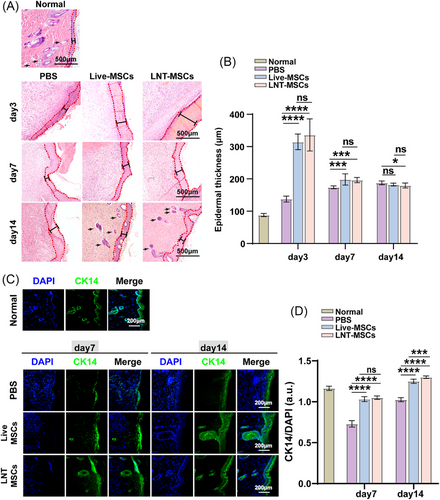
Keratin 14 (CK14) is primarily expressed in the basal cells of the proliferative compartment of the epidermis and can be used to evaluate the degree of re-epithelialisation. We detected the expression of CK14 in wound tissue on days 7 and 14. After treatment with LNT-MSCs or live-MSCs, the expression of CK14 was much higher than that after treatment with PBS, suggesting that LNT-MSCs and live-MSCs promoted re-epithelialisation. Furthermore, there were no notable distinctions observed between the LNT-MSC and live-MSC groups. (Figure 3C,D). Collectively, the LNT-MSCs promoted cutaneous wound healing by promoting re-epithelialisation.
3.3 Liquid nitrogen-treated UCMSCs promoted collagen deposition
Masson's trichrome staining was used to examine collagen deposition during wound healing. As compared to the PBS group, LNT-MSCs and live-MSCs showed greater collagen deposition and thicker collagen fibres on day 14. After treatment with LNT-MSCs, the collagen fibre arrangement was more regular and organised (Figure 4A,B). Immunohistochemical staining of collagen I showed that LNT-MSCs and live-MSCs treatment significantly increased the expression of collagen I, which suggests that LNT-MSCs and live-MSCs increase collagen deposition by upregulating collagen I expression (Figure 4C,D).
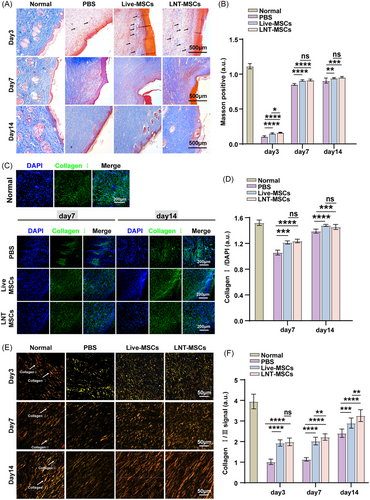
Then, sirius red staining was used to distinguish type I and type III collagen. On day 7, the ratio of type I to type III collagen increased in each group. The wounds treated with the LNT-MSC had a higher collagen I content. On day 14, the LNT-MSC treatment group exhibited mesh-like collagen organisation. In the LNT-MSC treatment group, collagen I/III ratios were similar to those in normal skin. Moreover, there were no significant discrepancies detected between the LNT-MSCs and live-MSCs groups (Figure 4E,F).
3.4 Liquid nitrogen-treated UCMSC stimulated angiogenesis in vivo
To investigate the effect of LNT-MSCs on angiogenesis in vivo, the expression levels of α-smooth muscle actin (α-SMA) and CD31 (platelet endothelial cell adhesion molecule-1) were determined characteristics and quantity of both vascular smooth muscle cells and endothelial cells involved in vascular regeneration on days 7 and 14. In wound beds treated with LNT-MSCs or live-MSCs, α-SMA and CD31-positive cells were observed. Quantification of α-SMA and CD31 fluorescence signals in the wound beds showed that LNT-MSCs treatment significantly upregulated the expression of α-SMA and CD31, compared to PBS treatment. The impact of LNT-MSCs was found to be similar to that of live-MSCs with no notable differences between the two (Figure 5). These results indicated that LNT-MSCs may provide a favourable microenvironment for wound healing by stimulating angiogenesis. LNT-MSCs exhibited comparable efficacy to live-MSCs in terms of angiogenesis stimulation.
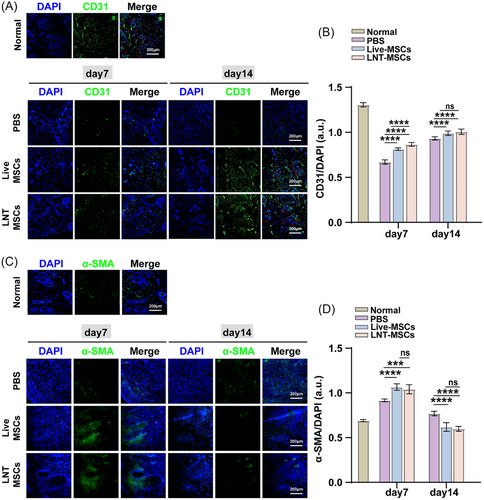
3.5 Liquid nitrogen-treated UCMSCs treatment induced immunomodulatory activity in vivo
To assess the in vivo immunomodulatory effects of LNT-MSCs, CD68 was utilised as the pan-macrophage marker and CD206 was used as the marker for M2-type macrophages. The CD68 and CD206 were detected in the wounded skin. Upon day 7 after surgery, CD68- and CD206-positive cells were significantly increased in granulation tissue treated with LNT-MSCs or live-MSCs. However, the LNT-MSCs and live-MSCs’ groups had significantly lower macrophage numbers on day 14 than the PBS group (Figure 6A–D).
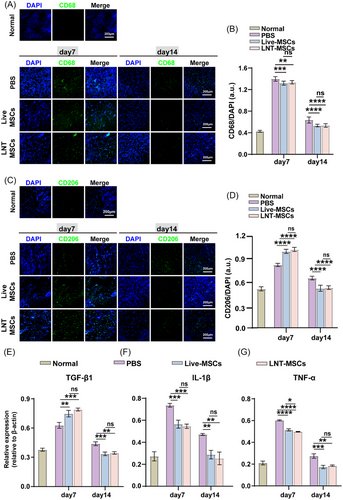
In addition, we analysed the relative gene expression of anti-inflammatory cytokine TGF-β1 and pro-inflammatory genes TNF-α and IL-1β in the wound bed by qRT-PCR. On day 7, the expression of TGF-β1, IL-1β and TNF-α was high in the wound bed. However, there was a significant reduction in their expression by day 14. TGF-β1 expression was significantly elevated by treatment with LNT-MSCs or live-MSCs on day 7, indicating that the LNT-MSCs and live-MSCs exhibited anti-inflammatory activity. Furthermore, the expression of IL-1β and TNF-α in the wound bed was significantly decreased by LNT-MSCs treatment compared to the PBS treatment group on both days 7 and 14 (Figure 6E–G). Collectively, these results indicate that LNT–MSCs exhibit immunomodulatory properties during wound healing.
3.6 Liquid nitrogen-treated UCMSCs promoted cell migration and proliferation in vitro
To further understand the underlying mechanism by which LNT-MSCs promote wound healing, we conducted in vitro experiments to assess the impact of LNT-MSCs on skin cell proliferation and migration. Fibroblasts (NIH/3T3 cells), endothelial cells (HUVEC) and keratinocytes (HaCaT cells) were co-cultured with LNT-MSCs or live-MSCs. Results from the scratch test revealed that both live-MSCs and LNT-MSCs significantly facilitated the migration of various cell types, including NIH/3T3 cells, HUVECs and HaCaT cells (Figure 7A–F). Analysis via CCK-8 assays showed that incubation with live-MSCs or LNT-MSCs significantly increased the numbers of NIH/3T3 cells, HUVECs, and HaCaT cells (Figure 7G–I).
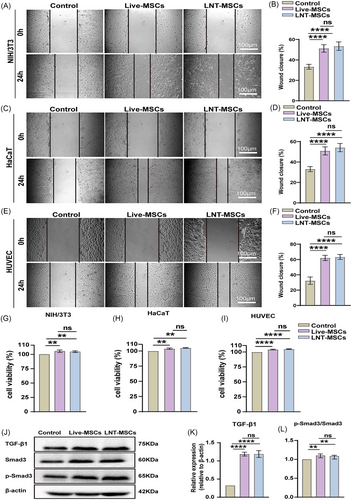
TGF-β/Smad signalling promotes cell migration, proliferation, and angiogenesis. LNT-MSCs significantly increased the expression of TGF-β1, Smad3 and p-Smad3 suggesting that LNT-MSCs activated the TGF-β/Smad signaling pathway (Figure 7J–L and Figure S4). No difference was observed between the respective effects of LNT-MSCs and live-MSCs.
3.7 Liquid nitrogen-treated UCMSCs promoted the polarisation of macrophages towards the M2 phenotype
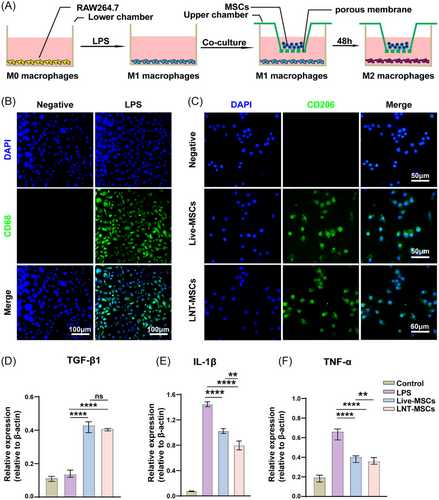
To determine whether LNT-MSCs played a role in promoting macrophages polarisation towards the M2 phenotype. We used LPS to mimic the inflammatory microenvironment of macrophages (Figure 8A). After LPS stimulation, RAW246.7 cells were polarised towards the M1 phenotype, with an increase in the percentage of CD68-positive cells. A significant increase in CD206-positive cells was observed with both LNT-MSCs and live-MSCs co-cultured with M1 macrophages, indicating a transformation of pro-inflammatory RAW264.7 cells to anti-inflammaging (Figure 8B,C). Following LPS treatment, the expressions of IL-1β and TNF-α were significantly increased. Treatment with LNT-MSCs led to a significant inhibition of TNF-α and IL-1β expression. The LNT-MSC treatment group exhibited significantly higher levels of TGF-1 expression than the control group. (Figure 8D–F). To investigate the impact of the cellular structure of MSCs on macrophage polarisation, we compared the effects of LNT-MSCs and lysed UCMSCs on macrophage polarisation. The results indicated that LNT-MSCs had a remarkable effect in promoting macrophage polarisation towards the M2 phenotype, in contrast to lysed UCMSCs (Figure S5).
4 DISCUSSION
We obtained dead but functional UCMSCs (LNT-MSCs) by rapidly immersing live-MSCs in liquid nitrogen. This approach eliminates the possibility of cell proliferation while temporarily maintaining the structural integrity of the cells and the expression of surface markers specific to MSCs. This is crucial for attracting host cells to the injury site and modulating the inflammatory microenvironment in the area.31 We then compared the therapeutic ability of LNT-MSCs and live-MSCs in the context of cutaneous wound repair. LNT-MSCs treatment promoted wound closure and new blood vessel formation. LNT-MSCs and live-MSCs exhibited similar morphology, cell surface markers and tissue damage repair abilities. Cryo-shocked cells are cells that are rapidly frozen in liquid nitrogen and lose their viability but retain their structure and function.30 The cryo-shocked AML cells maintain their bone marrow homing capability.30 Similarly, we present a simple technique to obtain inactive but still functional liquid nitrogen-treated umbilical cord MSCs. Our findings support the theory that cells subjected to rapid immersion in liquid nitrogen—a process known as cryo-shock—experience a loss of viability, but retain the structural integrity of the cell temporarily. The LNT-MSCs have potential applications in tissue engineering and regenerative medicine.
We investigated whether the intracellular protein and cytoskeletal structure of MSCs exert specific effects during wound healing and whether these effects are retained by LNT-MSCs. After exposure to liquid nitrogen, we observed that the cell membrane of LNT-MSCs exhibited a smoother appearance as compared to live-MSCs. We attributed this difference to the snap freeze process. It is likely that the snap freezing process can cause deformation of temperature-sensitive cell membrane proteins, while the use of cryoprotectants may have further contributed to the observed smoother surface of LNT-MSCs. Notably, the differences in the cell membrane characteristics of the LNT-MSCs may also indicate the alterations that occurred in response to the snap freeze process. Despite the morphological changes observed in the cell membrane, the surface markers CD29 and CD44 were detected. Stem cells adhere, homing ability, and proliferation are influenced by these surface markers.31 Intracellular MSC proteins are involved in modulating cell proliferation, inhibition of apoptosis and immunomodulation.32, 33 Our study investigated the use of LNT-MSCs for accelerating wound healing in rats with full-thickness skin defects. The therapeutic benefits observed from live-MSCs were also attained with LNT-MSCs. Unlike MSCs lysates, intact membrane structure and MSCs’ surface markers are retained on the LNT-MSCs, which might amplify their beneficial effects. And our results also showed that LNT-MSCs facilitated macrophage polarisation towards the M2 phenotype compared to lysed UCMSCs, indicating the importance of the cellular structure of MSCs in shaping the polarisation of macrophages.
It has been reported that cell membrane derived vesicles (nanoghosts) exhibit the functional properties of the original cells.34 Macrophage cell membrane-derived nanoghosts, retaining surface properties of macrophage, are able to reprogram M0 towards M2 macrophages.35 Nanoghosts produced from the cytoplasmic membranes of MSCs retain MSCs surface markers and have immunomodulatory capacity, which was similar to that of MSCs.34 Moreover, nanoghosts have shown promise as a means of delivering therapeutic agents. However, the nanoghosts can be difficult to produce in large quantities and have limitations with respect to stability and durability. The use of cryo-shocked cells allows for the production of a large number of cells in a single step, without the need for additional purification or functionalisation steps.
The process of wound healing involves skin cells proliferate and migrate to wound sites.36 After LNT-MSCs are applied to wounds, the cell membrane gradually ruptures, which can act as a source of bioactive molecules that promote tissue repair and regeneration. These molecules may include cytokines and growth factors, and can stimulate resident cells and recruit other cell types to the site of injury to promote tissue repair and regeneration.15, 37-39 In our study, fibroblasts (NIH/3T3 cells), endothelial cells (HUVEC) and keratinocytes (HaCaT cells) were co-cultured with LNT–MSCs, we observed LNT-MSCs promoted proliferation and migration of these cells. Therefore, the proliferation and migration of skin cells were accelerated by LNT-MSCs, so wound closure was accelerated. Besides, wound healing also relies on angiogenesis as it provides oxygen and nutrients to the injured area.13 We found that LNT-MSCs significantly upregulated expression of α-SMA and CD31, two essential markers for angiogenesis. Moreover, the cell surface receptors CD29 and CD44 were found to be present on LNT-MSCs. CD29, also known as integrin β1, is a transmembrane protein that mediates cell adhesion to the extracellular matrix (ECM). It binds to various ECM proteins such as fibronectin, laminin, and collagen, and is essential for cell migration, proliferation and survival.40 CD44 facilitates cell adhesion, migration, and differentiation, and contributes to the formation of new blood vessels. CD29 mediates the attachment of cells to the ECM, while CD44 binds to hyaluronic acid, which is essential for recruiting immune cells and promoting angiogenesis.39, 40 Additionally, after co-culturing LNT-MSCs with fibroblasts for 24 h, TGF-1, Smad3 and p-Smad3 were significantly upregulated. The TGF-β/Smad pathway is an important pathway involved in wound healing.41, 42 TGF-β1 promotes collagen and vascular endothelial growth factor secretion by stimulating the downstream factor Smad3, resulting in the acceleration of re-epithelialisation and angiogenesis.43
During the early stages of wound healing, inflammatory cells are extensively involved in damage repair.43 During repair and regeneration processes, MSCs produce multiple immunomodulatory agents and trophic factors.44 MSC-based clinical trials for cardiovascular therapy have primarily focused on the potential benefits of MSCs’ immunomodulatory and trophic properties, rather than their direct ability to generate new tissues.45 As a result of MSC stimulation, macrophages exhibit an M2-like phenotype and exhibit powerful anti-inflammatory properties.46, 47 The results suggested that LNT-MSCs induced macrophages to adopt the M2 phenotype. These data demonstrated that LNT-MSCs also have immunoregulatory functions. However, it is important to note that there are still many unanswered questions about the mechanisms underlying the effects of cryo-shock or liquid nitrogen treatment on cells. Further studies are needed to elucidate mechanisms and optimise use of LNT-MSCs.
Nevertheless, our experiments demonstrated that LNT-MSCs and live-MSCs were able to effectively promote wound healing. One clear advantage of using LNT-MSC is the potential for a scalable and manageable conventional pharmaceutical manufacturing process. The liquid nitrogen treatment process abrogates the proliferation and differentiation of stem cells while preserving the integrity of their cellular structure. It creates a ready-to-use cell bank for clinical applications and has the potential to serve as a cell bioengineering platform technology for different types of stem cells, making it a promising alternative to traditional stem cell transplants.
This strategy is convenient and uncomplicated from a manufacturing standpoint, and the procedure of cell cryopreservation in liquid nitrogen can be standardised. Isolated MSCs can be expanded and cryo-shocked to create a ready-to-use cell bank for clinical applications. It offers a promising alternative to conventional stem cell transplantation and has the potential to serve as a cell bioengineering platform technology for a wide range of stem cell types.
AUTHOR CONTRIBUTIONS
Y.Y., Y.Q.F., and M.R.H. conceived this project; J.X.D., S.Y.W., Z.Y.W., performed and analyzed the animal experiments; L.L., R.F.H., Y.X.W. Y.L. and T.Y. analyzed data and record experiments; Y.D.H. designed research and wrote the paper.
ACKNOWLEDGEMENTS
This work was supported by National Natural Science Foundation of China (No. 81871150, 32170865, and 82071634); Natural Science Foundation of Guangdong Province (No. 2022A1515012178); the Science and Technology Plan Project of Guangzhou (No. 202002030168); the Science and Technology Plan Project of Guangzhou (No. 202103030003); Guangdong Key Areas R&D Program (No. 2022B1111080007).
CONFLICT OF INTERESTS STATEMENT
The authors declared no conflicts of interest.




