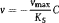Influence of Nonionic Surfactants on Fungal and Bacterial Degradation of Hexane
Abstract
Batch solubility and microcosm studies were conducted to examine the role of nonionic surfactants (Brij 30, Brij 35, Triton X-100, and Tween 80) on fungal and bacterial degradation of hexane, a model volatile organic compound with a high Henry's law constant. The apparent solubility of gas phase hexane was not observed as a linear correlation in respect to surfactant concentration due to the insufficient mass transfer at the gas–liquid interface. The hexane partitioning to micellar phase was related to the unique structure of surfactants with different lengths of hydrophobic alkyl or hydrophilic polyoxyethylene chains. Microcosm test results revealed that most tested surfactants below critical micelle concentration (CMC) improved the fungal and bacterial growth, but surfactants at high concentration (5 CMC) adversely affected biodegradation kinetics. The observed low hexane removal was possibly attributed to the toxicity of surfactants at elevated concentration, the preferential utilization of a surfactant as substrate, and the partitioning of hexane into micellar phase. The results from this study suggest that low concentrations of Brij 30 and Tween 80 are favorable to enhance both the solubility and bioavailability of hexane with fungi as working consortium, while Brij 30 and Triton X-100 below their CMC hold promising potential for bacterial degradation of hexane without having significant biodegradation of the surfactant.
Abbreviations
-
- B30
-
- Brij 30
-
- B35
-
- Brij 35
-
- CMC
-
- critical micelle concentration
-
- HLB
-
- hydrophile–lipophile balance
-
- T80
-
- Tween 80
-
- TBAB
-
- trickle bed air biofilter
-
- TX100
-
- Triton X-100
-
- VOC
-
- volatile organic compound
1 Introduction
Due to stringent air quality regulations, there is an increasing demand to control the emission of volatile organic compounds (VOCs) from their various sources 1. Hexane, an organic solvent commonly used in industry, is highly volatile and can be present in air at elevated concentrations, posing an exposure risk to humans 2, 3. Inhalation of a high level of hexane has been linked to nervous system damage and cancer development 4. To reduce the risks to humans and the environment, biological treatment technologies (e.g., biotrickling filters, bioscrubbers, and two-liquid phase systems) have emerged as environmentally friendly and economic options for VOC removal in polluted air 5-7. However, the biological removal of VOCs is challenged by high hydrophobicity and limited mass transfer of hexane in aqueous phase, where biodegradation by microconsortia occurs 8-10.
To increase the bioavailability of hydrophobic hexane, surfactants, which have both hydrophilic and hydrophobic groups, are considered as a means to enhance solubility and biodegradation. Among various surfactants, nonionic surfactants have been commonly used in the biodegradation of hydrophobic compounds, as they exhibit low bacterial toxicity compared to anionic surfactants 11, 12. Hassan and Sorial 9, 13, 14 evaluated the effects of nonionic surfactants (Triton X-100 and Tomadol® 25-7) on hexane degradation in a trickle bed air biofilter (TBAB) system, where they observed improved hexane degradation at pH 4, which is a favorable condition for fungi growth. Other studies of hexane biodegradation by filamentous fungi without the presence of surfactants (e.g., Aspergillus niger, Cladosporium, and Fusarium spp.) have also reported improved hexane removal, attributed to the facilitated mass transfer of hexane through the aerial structures of fungi that provide a large contact area for the biosorption and uptake of hexane 15-17. In addition, several fungal species are known to tolerate abrupt changes in pH and moisture content known to occur during biofilter system operation 9, 18.
Although surfactant addition may greatly improve hexane removal, there are many uncertainties in the application of surfactants to biofilter systems for this purpose, which include surfactant toxicity to microorganisms and preferential degradation of surfactants over hexane by the microbial consortia. Even though many previous studies have explored surfactant-enhanced bioremediation of hydrophobic organic compounds in polluted air 9, 13, 14 or contaminated soils 19-21, a knowledge gap still exists concerning the combined role of nonionic surfactants on the solubility and fungal degradation kinetics of hexane. Optimization of biofilter system operation and performance for the treatment of hexane (and other hydrophobic VOCs) necessitates understanding the response of microbial consortia to nonionic surfactant addition in the system.
In this study, the influence of nonionic surfactants on hexane solubility and fungal degradation kinetics was investigated. First, commonly used nonionic surfactants (Brij 30, Brij 35, Triton X-100, and Tween 80) were investigated to determine their respective impact on the aqueous solubility of hexane as a function of surfactant concentration. Using results from the solubility tests, microcosm studies were conducted to elucidate the nonionic surfactant effects on the fungal degradation kinetics of hexane, where the experimental results were fitted into solubility and degradation kinetic models to investigate the influence of surfactants on both metabolic activity of microorganisms and biodegradation rate of hexane. As fungi are typically not solely present in engineered biofiltration systems, the bacterial degradation of hexane in the presence of surfactants was also investigated and compared to fungal degradation kinetics.
2 Materials and methods
2.1 Chemicals
Hexane (99% purity, Fisher Scientific) was used as received. Four nonionic surfactants, Brij 30 (B30), Brij 35 (B35), Triton X-100 (TX100), and Tween 80 (T80), were purchased from Sigma–Aldrich and used without further purification. The properties of the tested surfactants are outlined in Tab. 1, as reported in previous studies. The hydrophile–lipophile balance (HLB) defines the proportion of hydrophilic/lipophilic groups in a surfactant molecule 22. A low HLB value indicates a hydrophobic characteristic for surfactants, and vice versa. The critical micelle concentration (CMC) used in this study was 12.7, 110.2, 143.8, 17.0 mg L−1 for B30, B35, TX100, and T80, respectively 11, 19, 20, 23-30.
| Trade name | Structurea) | Formulaa) | MWa) | HLBb) | CMC (mg L−1)c) | Molar ratiod) |
|---|---|---|---|---|---|---|
| B30 | 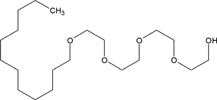 |
C12H25 (OC2H4)4OH | 362 | 9.5 | 12.7 | 5.03 × 10−4 |
| TX100 | 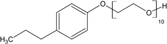 |
C8H17C6H4(OC2H4)10OH | 625 | 13.5 | 143.8 | 4.04 × 10−5 |
| B35 | 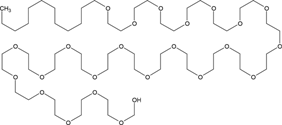 |
C12H25 (OC2H4)23OH | 1200 | 16.9 | 110.2 | 2.26 × 10−6 |
| T80 | 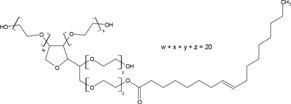 |
C18H37C6H9O5(OC2H4)20OH | 1309 | 15.0 | 17.0 | 3.93 × 10−4 |
- a) Molecular information of nonionic surfactants tested refers to Laha and Luthy 20, Grimberg et al. 23, Prak and Pritchard 27, Tiehm 28, and Guha and Jaffe 40.
- b) HLB values refer to Griffin 22, Grimberg et al. 23, and Slinde and Flatmark 46.
- c) CMC values are selected based on previously reported values 11, 19, 20, 23-30.
- d) Molar ratio = (molar of hexane solubilized at CMC − molar of hexane solubilized without surfactant)/molar of surfactant at CMC.
2.2 Solubility tests
Aqueous hexane solubility tests were conducted in 125 mL serum bottles (Wheaton Industries, NJ) with aluminum cap and septum. Stock surfactant solutions (0, 0.5, 1.0, 2.0, 5.0, and 10.0 CMC) were prepared and 50 mL was aliquoted into three replicate serum bottles (Ntotal = 72). To introduce hexane for solubility assessment, gas-phase hexane was generated in a static dilution bottle (Kimble & Chase, NJ) at room temperature and then transferred by a gas tight syringe (Valco Instruments, TX) into serum bottles, providing an initial 250 ppmv headspace concentration 9. Then, the serum bottles were placed into a tumbler at 12 rpm for mixing. Sampling time was at 1, 3, 6, 12, 24, 36, 48, 72, and 96 h.
2.3 Fungal and bacterial culture preparation
Both fungal and bacterial cultures were obtained from a TBAB system at the University of Cincinnati. Effluent samples containing fungal and bacterial biomass were collected during backwashing period of the filters. Zehraoui et al. 31 characterized the species of microorganisms in the TBAB system. They identified Fusarium spp. and A. niger as the dominant species for fungi and Mycobacterium sp. for bacteria using the 16S rDNA and 18S rDNA PCR and sequencing analyses 31. Collected biomass samples from each filter were centrifuged and grown in batch reactors at pH 4 (fungi promoting) and pH 7 (bacteria promoting), respectively, until steady state growth was achieved. Gas phase hexane was added to the reactors as the sole carbon source using a syringe pump (KD Scientific, MA) at a loading rate of 5.37 g m−3 · h−1 through a gas diffuser submerged in the reactor. A micronutrient solution of mineral salts and buffered nutrients was also added to the reactors following the chemical composition by Hassan and Sorial 13. The detailed chemical composition of the micronutrient solution is provided in Supporting Information Tab. S1. After reaching steady-state growth, the biomass from both fungal and bacterial reactors was harvested and washed with phosphate buffer solution (pH 4 and 7 for fungi and bacteria, respectively), so frozen culture stock solutions could be prepared for microcosm tests.
2.4 Microcosm study
In order to elucidate the role of nonionic surfactants on hexane degradation kinetics, microcosm tests were conducted using 125 mL serum bottles. A working volume of 50 mL was utilized for the tests, which included microbial seed culture, mineral salts, nutrients, and surfactant at various concentrations (0, 0.5, and 5.0 CMC). Student's t-test conducted using JMP statistical software suggested that the difference in gas phase hexane solubility at 0.5 and 5.0 CMC was significant. Solution pH was adjusted to pH 4 for fungal biomass experiments and pH 7 for bacterial biomass degradation tests and 1 mL of culture stock solution was added to each bottle to initiate the trials. Then, the prepared sample bottles were placed in a rotary tumbler at 12 rpm and the gas-phase hexane concentrations in the bottles were monitored every 2 days for 14–18 days according to previously reported acclimation times for hexane degradation in TBAB systems 9, 14, 31, 32. All microcosm tests were conducted at room temperature (20°C) to be consistent with the common operational temperature of TBAB in other studies 9, 13, 14.
2.5 Analysis
A gas chromatograph (Hewlett-Packard 6890) equipped with a mass selective detector and HP-624 column (30 m × 250 μm × 0.25 μm) was used to measure the concentration of hexane in the gas phase. For the analysis, the flow rate of the carrier gas (helium, 99.999%) was 6.7 mL min−1. The set point temperature of the heater was at 260°C and pressure was at 2.82 psi. Detector and injector temperatures were held at 250 and 120°C, respectively. Standard gas-phase solutions for hexane were prepared by spiking different amount of liquid hexane into the static dilution bottles. Once the hexane completely vaporized in the dilution bottles, the gas-phase sample was transferred to the GC/MS injector using a 0.5 mL gas tight syringe. Evaluation of serum bottle hexane concentrations followed the same injection method.
3 Results and discussion
3.1 Hexane solubility kinetics
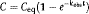 (1)
(1)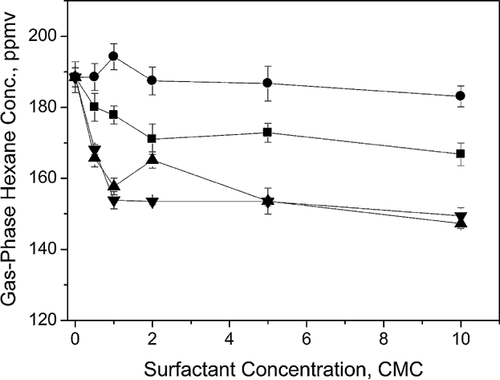
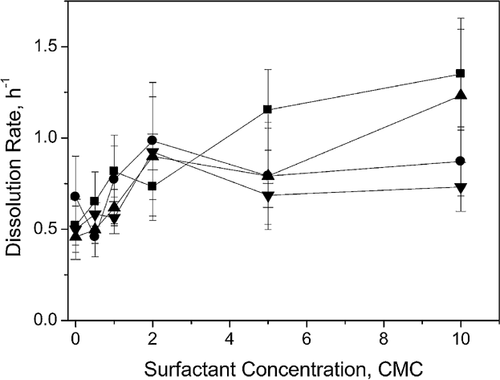
Table 1 includes the molar solubility ratio of tested surfactants (B35 < TX100 < T80 < B30) at their respective CMC. The ratio was determined by calculating the molar concentration differences of hexane solubilized in a surfactant solution at CMC and in a solution without a surfactant divided by the molar concentration of the surfactant solution. Differences in solubility enhancement by the tested surfactants were attributed to their unique molecular structures which affects hexane partitioning into the micelles. As shown in Tab. 1, B30, TX100, and B35 all have one hydrophilic polyoxyethylene sequence and a short tail of hydrophobic alkyl chain. T80, on the other hand, possesses several polyoxyethylene chains and a long hydrophobic alkyl tail. The solubility enhancements by the former three surfactants correlated inversely with the HLB values. A higher HLB value indicates a more hydrophilic micelle and thus, a smaller hydrophobic core in the micelle, resulting in reduced hexane partitioning into the micelle. Grimberg et al. 23 observed the same solubility trend when evaluating surfactant HLB values for hydrophobic phenanthrene. Despite this trend, T80 has a relatively high HLB value of 15 (Tab. 1); however, its long hydrophobic alkyl chain can result in more hexane partitioned into the micellar phase, therefore, facilitating the solubilization of hexane.
The effect of surfactants on the dissolution rate of gas-phase hexane is controlled by two steps: mass transfer and saturation 23. The number of saturation sites is a linear function of the surfactant concentration. Therefore, as described previously, the observed nonlinear relationship between solubility rate of hexane and surfactant concentration was due to the insufficient mass transfer of hexane from headspace to solution. The higher dissolution rates of hexane in B30 and TX100 than those of B35 and T80 are probably attributed to the relatively smaller molecular size of the surfactants, which is related to a higher diffusivity in water resulting in faster mass transfer process 35.
3.2 Microcosm study
The effects of nonionic surfactants on fungal and bacterial growth utilizing hexane were investigated with a serum bottle-based microcosm study. The microcosm study results showed that the amount of hexane in the headspace decreased rapidly in 9 days and then remained unchanged for both cultures (Supporting Information Fig. S5; selected surfactants with high hexane removal are shown for representative data). Figure 3 shows the biomass growth in the serum bottles and corresponding hexane removal per unit biomass in the presence of tested surfactants. After injection, the gas phase hexane firstly dissolved into the aqueous solution resulting in the reduction of headspace hexane concentration. Therefore, hexane removal due to biodegradation was calculated by subtracting the reduced concentration at designated operation time from the solubility concentration at equilibrium. Overall, the fungi and bacteria responded differently to the addition of tested surfactants, despite optimized growth pH. For example, the hexane removal per unit mass of fungal culture without surfactant was 3.2 mg g−1, with a maximum removal of 4.5 mg g−1 in the presence of 0.5 CMC B30 during the experimental period. For bacterial degradation, the final hexane removal at pH 7 in the absence of surfactant was 22.3 mg g−1, whereas addition of 0.5 CMC B30, B35, or TX100 increased the removal up to 58.4 mg g−1. Overall improvement in degradation was attributed to the surfactant-assisted mass transfer of hexane into the aqueous phase for bio-uptake. The difference in hexane removal between fungi and bacteria could be due to the lower growth rate of fungi as compared to bacteria, which is consistent to previous observations 36. Another possible reason for the low hexane removal was temperature effects. Iranmanesh et al. 3 recently reported an optimum temperature of 36.5°C for fungal degradation of hexane. The removal was reduced to 32.6% at a temperature of 25°C. The microcosm tests in this study were conducted at room temperature (20°C) which likely diminishes fungal metabolic activity.
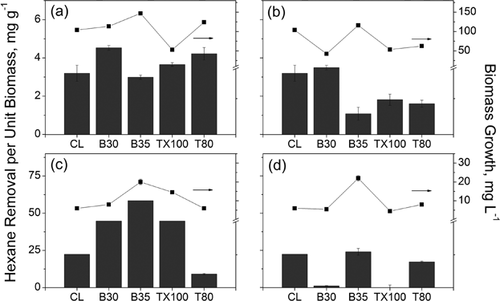
For fungal degradation of hexane, the results in Fig. 3a suggest that 0.5 CMC B30 and T80 increased the hexane removal while B35 and TX100 showed no improvement to the degradation process. Higher fungal growth (7.0–40.0 mg L−1 higher biomass than control) was observed for all surfactants except for TX100 as it exhibited toxic effects to the fungal culture 37. However, TX100 can be a good candidate when seeking for comparable hexane degradation at a much lower biomass accumulation. It is further noted that B35 with the lowest observed solubility enhancement yielded the highest biomass growth, followed by B30 and T80. This may indicate that fungi utilized the surfactants, especially B35, as carbon source 38 resulting in greater fungal growth 39. At 5 CMC, the hexane biodegradation and biomass growth (except for B35) were inhibited due to the toxicity of the surfactants as well as increased partitioning of hexane into surfactant micelle phase (Fig. 3b) 40. The large amount of surfactant molecules likely reshaped the cellular structures of fungi by interacting with its lipid component or reacting with enzymes and other essential proteins in the fungal cell 20, 41. As shown in Fig. 3b, the dysfunctional ability of TX100 and T80 for fungal growth was lower than that of B30 which, however, yields a comparable hexane removal to the control without surfactants. In contrast, fungi easily utilized B35 at high concentrations over hexane as carbon source in aqueous solution, leading to reduced hexane degradation. This preferential consumption of B35 as an abundant carbon source resulted in a slightly higher biomass growth than that of control in Fig. 3b.
Figure 3c shows that B30, B35, and TX100 at low concentrations (0.5 CMC) all enhanced the bacterial degradation of hexane. The increased hexane removal was attributed to increased mass transfer into the aqueous facilitated by the nonionic surfactants which worked as transfer terminals for hexane. Considering similar biomass growth in the presence of T80 compared to that of B30, the reduced hexane removal by T80 was possibly due to preferential utilization of T80 over hexane as a substrate for bacterial growth 42. The highest biomass yield under B35 was likely the result of consumption of the surfactant. In addition, TX100 showed facilitation, instead of inhibition, to both biomass growth and biodegradation. As compared to TX100, B30 promoted less biomass growth for the same removal efficiency, suggesting B30 is a better candidate for biofiltration systems for hexane removal with less backwashing. In Fig. 3d, the presence of surfactants at high concentrations (5 CMC) caused adverse effects on the bacterial biodegradation process. The adverse effects were more evident for B30 and TX100 even though both surfactants facilitated the gas-phase hexane dissolved in water. For B35, higher biomass growth was also observed, which again implies B35 was utilized by bacteria as a preferential substrate. A slightly increased biomass growth in the presence of T80 further confirms the utilization of T80 as substrate. The higher biomass yield with B35 and T80 subsequently consumed more hexane than that of B30 and TX100 by co-metabolism or biosorption 39. Since 5 CMC surfactants did not inhibit biomass growth, the effect of surfactant toxicity was excluded for bacterial degradation of hexane. The decreased hexane bioavailability under high surfactant concentration was possibly due to the increased partitioning of hexane into surfactant micelle phase as a function of micelle quantity, which limited direct mass transfer from the micelle to microorganisms. Guha and Jaffe 40 reported similar observations for the biodegradation of a model polycyclic aromatic hydrocarbon (i.e., phenanthrene). In their microcosm studies, they observed a slower degradation rate of phenanthrene with surfactant concentrations beyond CMC. They concluded that bioavailability of phenanthrene decreased with increasing surfactant concentrations due to mass transfer limitations of the partitioned phenanthrene from surfactant micelles to the bacteria.
3.3 Hexane degradation kinetics
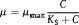 (2)
(2)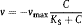 (3)
(3)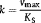 (5)
(5)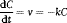 (6)
(6)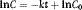 (7)
(7)Since the aqueous hexane concentration (<1.3 mg L−1 at complete dissolution) was much smaller than the reported KS value (16 mg L−1) 45, the degradation result as a function of time was recorded and then fitted to the above linear relationship to obtain the pseudo first-order reaction rate constant k. Figure 4 shows the degradation kinetics of hexane by fungi (pH 4) and bacteria (pH 7) in the presence of tested nonionic surfactants. Overall, low surfactant concentrations increased the hexane degradation rates compared to controls without surfactants. However, the influence of high surfactant concentrations on degradation kinetics was more complicated. As compared to fungi, higher overall degradation rates were observed by bacteria which are possibly due to the larger cell population per biomass weight and the lower optimal metabolic temperature for bacterial growth.
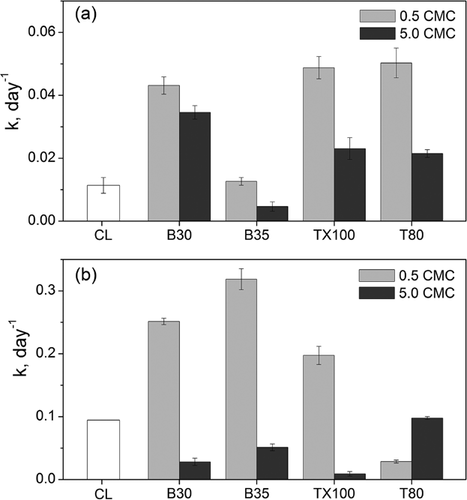
In Fig. 4a, the comparable degradation rates between 0.5 CMC B35 and control were consistent with hexane removal in Fig. 3a. However, in Fig. 4b, 0.5 CMC B35 with the lowest solubility molar ratio (Tab. 1) exhibited the highest degradation rate for hexane by bacteria. The accelerated hexane degradation was likely due to the utilization of B35 as a carbon source which supported higher biomass growth and subsequently accelerated degradation of hexane. However, at higher concentrations, B35 outcompetes hexane for bio-uptake, thus diminishing the hexane biodegradation rate in comparison. The similar fungal degradation kinetics for 0.5 CMC B30, TX100, and T80 parallel the observed hexane solubility rates in Fig. 2. The same correlation was observed for bacterial degradation kinetics for B30 and TX100. As compared to surfactants below CMC, the reduced degradation rates at 5 CMC (except for T80 for bacteria) mirror the inhibited biomass growth in Fig. 3b and d indicating the adverse effect of high surfactant loading rates which may be associated with surfactant toxicity or reduced mass transfer of hexane due to strong partitioning of hexane into surfactant micelle phase. However, 5 CMC B30, TX100, and T80 still enhanced hexane degradation rates by fungi. As for T80, its higher level of hexane dissolution at 5 CMC (153.5 ± 1.3 ppmv in headspace) compared to that at 0.5 CMC (168.1 ± 1.4 ppmv in headspace) resulted in the faster degradation rate of hexane by bacteria. The slow hexane removal by 0.5 CMC T80 was consistent to the observation of reduced hexane degradation in Fig. 3c and d, although similar biomass growth was observed at 0.5 and 5.0 CMC T80 conditions, which again suggests potential utilization of T80 as a substrate for bacterial growth 42.
Overall, the surfactants (B30 and T80) tested in this study provided optimal hexane removal by fungal consortia at 0.5 CMC, whereas concentrations of B30 and TX100 below their CMC are better options for degradation of hexane if considering bacteria. Although B35 showed high hexane removal, B35 may not be the best surfactant for TBAB systems as it is easily consumed by fungi and bacteria producing high amount of biomass. Long-term monitoring of the fungal degradation of hexane in the presence of tested surfactants is needed. In addition, both fungal and bacterial consortia in the TBAB system will be present as biofilm grown on the supporting media of the system. Therefore, hexane degradation by the mixed consortium needs to be studied as attached biofilm and the influence of nonionic surfactant on biofilm formation and growth kinetics needs to be further determined with a fundamental understanding of interactions among microorganisms, VOCs, and surfactants.
4 Concluding remarks
The present study elucidated the role of nonionic surfactants on the solubility and degradation kinetic of a model VOC, hexane. Batch solubility tests prior to biodegradation tests revealed that the presence of surfactants even below CMC enhanced hexane solubility. Among tested surfactants, T80 showed the highest apparent solubility enhancement for hexane, while B35 showed the lowest in aqueous solutions. In the microcosm study, overall, fungi had lower hexane degradation rates than bacteria, suggesting that maintaining optimum temperature may be needed to enhance the fungal degradation of VOCs. The addition of nonionic surfactants showed promise to enhance both the solubility and bioavailability of hexane in the biofilter systems under different microconsortia conditions. Overall, the study results advance our understanding of process parameters useful toward improving contaminant reduction by biofilter systems for VOCs with low solubility.
Acknowledgments
This work was supported by National Science Foundation under award no. CBET 0852803. Authors gratefully acknowledge Dr. Ashraf Hassan for his help in culture acquisition from biofilters and gas-phase hexane measurements with GC.
The authors have declared no conflicts of interest.