Expedient Cyano-hydroxylation of Alkenes Enabled by Halogen Atom Transfer Induced Radical Ring-Opening Elaboration of 3-Bromo-isoxazoline Cycloadducts
Hui Wang
Technical Institute of Fluorochemistry (TIF), Institute of Advanced Synthesis (IAS), School of Chemistry and Molecular Engineering, Nanjing Tech University, 30 South Puzhu Road, Nanjing, Jiangsu, 211816 China
These authors contributed equally to this work.
Search for more papers by this authorQing Chen
Technical Institute of Fluorochemistry (TIF), Institute of Advanced Synthesis (IAS), School of Chemistry and Molecular Engineering, Nanjing Tech University, 30 South Puzhu Road, Nanjing, Jiangsu, 211816 China
These authors contributed equally to this work.
Search for more papers by this authorShuhui Wang
Technical Institute of Fluorochemistry (TIF), Institute of Advanced Synthesis (IAS), School of Chemistry and Molecular Engineering, Nanjing Tech University, 30 South Puzhu Road, Nanjing, Jiangsu, 211816 China
Search for more papers by this authorCorresponding Author
Cheng-Qiang Wang
Technical Institute of Fluorochemistry (TIF), Institute of Advanced Synthesis (IAS), School of Chemistry and Molecular Engineering, Nanjing Tech University, 30 South Puzhu Road, Nanjing, Jiangsu, 211816 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Chao Feng
Technical Institute of Fluorochemistry (TIF), Institute of Advanced Synthesis (IAS), School of Chemistry and Molecular Engineering, Nanjing Tech University, 30 South Puzhu Road, Nanjing, Jiangsu, 211816 China
State Key Laboratory of Material-Oriented Chemical Engineering, Nanjing Tech University, 30 South Puzhu Road, Nanjing, Jiangsu, 211816 China
E-mail: [email protected]; [email protected]Search for more papers by this authorHui Wang
Technical Institute of Fluorochemistry (TIF), Institute of Advanced Synthesis (IAS), School of Chemistry and Molecular Engineering, Nanjing Tech University, 30 South Puzhu Road, Nanjing, Jiangsu, 211816 China
These authors contributed equally to this work.
Search for more papers by this authorQing Chen
Technical Institute of Fluorochemistry (TIF), Institute of Advanced Synthesis (IAS), School of Chemistry and Molecular Engineering, Nanjing Tech University, 30 South Puzhu Road, Nanjing, Jiangsu, 211816 China
These authors contributed equally to this work.
Search for more papers by this authorShuhui Wang
Technical Institute of Fluorochemistry (TIF), Institute of Advanced Synthesis (IAS), School of Chemistry and Molecular Engineering, Nanjing Tech University, 30 South Puzhu Road, Nanjing, Jiangsu, 211816 China
Search for more papers by this authorCorresponding Author
Cheng-Qiang Wang
Technical Institute of Fluorochemistry (TIF), Institute of Advanced Synthesis (IAS), School of Chemistry and Molecular Engineering, Nanjing Tech University, 30 South Puzhu Road, Nanjing, Jiangsu, 211816 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Chao Feng
Technical Institute of Fluorochemistry (TIF), Institute of Advanced Synthesis (IAS), School of Chemistry and Molecular Engineering, Nanjing Tech University, 30 South Puzhu Road, Nanjing, Jiangsu, 211816 China
State Key Laboratory of Material-Oriented Chemical Engineering, Nanjing Tech University, 30 South Puzhu Road, Nanjing, Jiangsu, 211816 China
E-mail: [email protected]; [email protected]Search for more papers by this authorComprehensive Summary
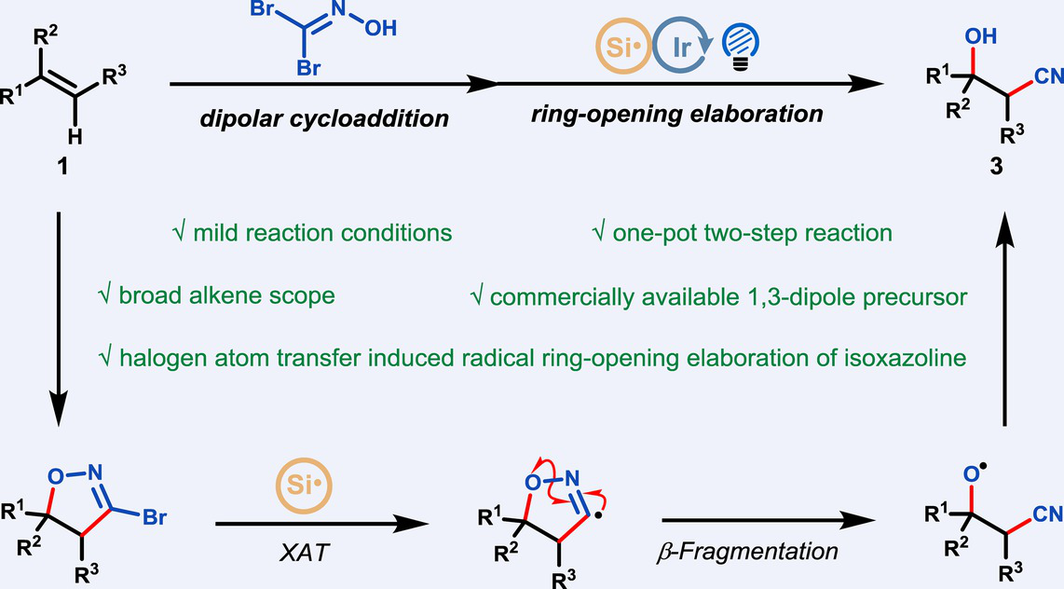
Herein, we present a highly efficient one-pot, two-step synthesis of β-hydroxy nitrile scaffolds, which possess both significant synthetic value and notable biological activity, starting from readily accessible alkenes. This methodology relies crucially on the seamless integration of a highly regioselective (3+2) cycloaddition reaction, employing the commercially available 1,1-dibromoformaldoxime as the 1,3-dipole precursor, with a subsequent halogen atom transfer-induced radical ring-opening elaboration of the resulting 3-bromo-2-isoxazoline cycloadducts. This protocol is featured by mild reaction conditions, broad alkene scope and various derivatizations of the obtained cyano-hydroxylation products, offering a versatile and practical pathway to accessing multi-functionalized molecules.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202500233-sup-0001-supinfo.pdfPDF document, 5.3 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1Selected book chapter and reviews, see: (a) Jacques, B.; Muñiz, K. Palladium Catalysis for Oxidative 1,2-Difunctionalization of Alkenes. Catalyzed Carbon-Heteroatom Bond Formation, John Wiley & Sons, Ltd., Hoboken, 2010, pp. 119–135;
10.1002/9783527633388.ch4 Google Scholar(b) Sauer, G. S.; Lin, S. An Electrocatalytic Approach to the Radical Difunctionalization of Alkenes. ACS Catal. 2018, 8, 5175–5187; (c) Fu, X.; Zhao, W. Progress in Difunctionalization of Alkenes. Chin. J. Org. Chem. 2019, 39, 625–647; (d) Li, Z.-L.; Fang, G.-C.; Gu, Q.-S.; Liu, X.-Y. Recent advances in copper-catalysed radical involved asymmetric 1,2-difunctionalization of alkenes. Chem. Soc. Rev. 2020, 49, 32–58; (e) Jiang, H.; Studer, A. Intermolecular radical carboamination of alkenes. Chem. Soc. Rev. 2020, 49, 1790–1811; (f) Liu, Y.; Liu, H.; Liu, X.; Chen, Z. Recent Advances in Photoredox-Catalyzed Difunctionalization of Alkenes. Catalysts 2023, 13, 1056; (g) Zhou, Z.-L.; Zhang, Y.; Cui, P.-Z.; Li, J.-H. Photo-/Electrocatalytic Difunctionalization of Alkenes Enabled by C–H Radical Functionalization. Chem. Eur. J. 2024, 30, e202402458.
- 2Selected reviews, see: (a) Zhu, S.; Zhao, X.; Li, H.; Chu, L. Catalytic three-component dicarbofunctionalization reactions involving radical capture by nickel. Chem. Soc. Rev. 2021, 50, 10836–10856; (b) Wickham, L. M.; Giri, R. Transition Metal (Ni, Cu, Pd)-Catalyzed Alkene Dicarbofunctionalization Reactions. Acc. Chem. Res. 2021, 54, 3415–3437; (c) Jose, J.; Mathew, T. V. Recent Advances in the 1,2-Diarylation of Alkenes. Adv. Synth. Catal. 2023, 365, 4334−4358; (d) Luo, Y.-C.; Xu, C.; Zhang, X. Nickel-Catalyzed Dicarbofunctionalization of Alkenes. Chin. J. Chem. 2020, 38, 1371−1394; (e) Lu, L.; Qiu, X. Recent Progress in Radical Involved Alkene Dialkylation. Chin. J. Org. Chem. 2024, 44, 1701–1718.
- 3Selected reviews and example, see: (a) Cresswell, A. J.; Eey, S. T.-C.; Denmark, S. E. Catalytic, Stereoselective Dihalogenation of Alkenes: Challenges and Opportunities. Angew. Chem. Int. Ed. 2015, 54, 15642–15682; (b) Bock, J.; Guria, S.; Wedek, V.; Hennecke, U. Enantioselective Dihalogenation of Alkenes. Chem. Eur. J. 2021, 27, 4517–4530; (c) Giri, R.; Zhilin, E.; Kissling, M.; Patra, S.; Fernandes, A. J.; Katayev, D. Visible-Light-Mediated Vicinal Dihalogenation of Unsaturated C−C Bonds Using Dual-Functional Group Transfer Reagents. J. Am. Chem. Soc. 2024, 146, 31547−31559.
- 4Selected book chapter and reviews, see: (a) Noe, M. C.; Letavic, M. A.; Snow, S. L. Asymmetric Dihydroxylation of Alkenes. Organic Reactions, John Wiley & Sons, Ltd., Hoboken, 2005, pp. 109–625;
10.1002/0471264180.or066.02 Google Scholar(b) Kolb, H. C.; VanNieuwenhze, M. S.; Sharpless, K. B. Catalytic Asymmetric Dihydroxylation. Chem. Rev. 1994, 94, 2483–2547; (c) Bataille, C. J. R.; Donohoe, T. J. Osmium-free direct syn-dihydroxylation of alkenes. Chem. Soc. Rev. 2011, 40, 114–128; (d) Bag, R.; De, P. B.; Pradhan, S.; Punniyamurthy, T. Recent Advances in Radical Dioxygenation of Olefins. Eur. J. Org. Chem. 2017, 5424–5438.
- 5Selected reviews, see: (a) Tao, Z.-L.; Denmark, S. E. Catalytic, Enantioselective Diamination of Alkenes. Synthesis 2021, 53, 3951–3962; (b) Kumar, R.; Khanna, Y.; Kaushik, P.; Kamal, R.; Khokhar, S. Recent Advancements on Metal-Free Vicinal Diamination of Alkenes: Synthetic Strategies and Mechanistic Insights. Chem. Asian J. 2023, 18, e202300017; (c) Kumar, R. Transition-Metal-Catalyzed 1,2-Diaminations of Olefins: Synthetic Methodologies and Mechanistic Studies. Chem. Asian J. 2024, 19, e202300705.
- 6Selected reviews, see: (a) Suginome, M.; Ito, Y. Transition-Metal- Catalyzed Additions of Silicon−Silicon and Silicon−Heteroatom Bonds to Unsaturated Organic Molecules. Chem. Rev. 2000, 100, 3221–3256; (b) Beletskaya, I.; Moberg, C. Element−Element Additions to Unsaturated Carbon−Carbon Bonds Catalyzed by Transition Metal Complexes. Chem. Rev. 2006, 106, 2320–2354; (c) Burks, H. E.; Morken, J. P. Catalytic enantioselective diboration, disilation and silaboration: new opportunities for asymmetric synthesis. Chem. Commun. 2007, 4717–4725; (d) Ji, C.; Gao, D. Recent Advances in Catalytic Asymmetric Synthesis of Chiral 1,2-Bis(boronic) Esters. Chin. J. Org. Chem. 2024, 44, 1385–1402.
- 7(a) Hoye, T. R.; Ye, Z.; Yao, L. J.; North, J. T. Synthesis of the C2-Symmetric, Macrocyclic Alkaloid, (+)-Xestospongin A and Its C(9)-Epimer, (-)-Xestospongin C: Impact of Substrate Rigidity and Reaction Conditions on the Efficiency of the Macrocyclic Dimerization Reaction. J. Am. Chem. Soc. 1996, 118, 12074−12081; (b) Pospíšil, J.; Markó, I. E. Total Synthesis of Jerangolid D. J. Am. Chem. Soc. 2007, 129, 3516−3517; (c) Wadhwa, K.; Verkade, J. G. P(i-PrNCH2CH2)3N as a Lewis Base Catalyst for the Synthesis of β-Hydroxynitriles Using TMSAN. J. Org. Chem. 2009, 74, 5683–5686; (d) Pohjakallio, A.; Pihko, P. M.; Liu, J. Base-Catalyzed Isomerization of 2-Isoxazolines Enables a Two-Step Enantioselective Synthesis of β-Hydroxynitriles from Enals. J. Org. Chem. 2010, 75, 6712–6715; (e) Fan, Y.-C.; Dua, G.-F.; Sun, W.-F.; Kang, W.; He, L. N-Heterocyclic carbene-catalyzed cyanomethylation of aldehydes with TMSAN. Tetrahedron Lett. 2012, 53, 2231−2233.
- 8Selected examples, see: (a) Chini, M.; Crotti, P.; Favero, L.; Macchia, F. Easy Direct Stereo- and Regioselective Formation of β-Hydroxy Nitriles by Reaction of 1,2-Epoxides with Potassium Cyanide in the Presence of Metal Salts. Tetrahedron Lett. 1991, 32, 4775−4778;
(b) Yamasaki, S.; Kanai, M.; Shibasaki, M. Novel Multiaction of Zr Catalyst: One-Pot Synthesis of β-Cyanohydrins from Olefins. J. Am. Chem. Soc. 2001, 123, 1256−1257;
(c) Kiasat, A. R.; Ayashia, N.; Fallah- Mehrjardi, M. Silica-Bound 3-{2-[Poly(ethylene Glycol)]ethyl}-Substituted 1-Methyl-1H-imidazol-3-ium Bromide: A Recoverable Phase- Transfer Catalyst for Smooth and Regioselective Conversion of Oxiranes to β-Hydroxynitriles in Water. Helv. Chim. Acta 2013, 96, 275−279;
10.1002/hlca.201200192 Google Scholar(d) Shahiri-Haghayegh, M.; Azizi, N.; Heidarzadeh, F. Greener and Additive-Free Ring Opening of Epoxides by All-in-One Choline Systems. ChemistrySelect 2020, 5, 14538−14542.
- 9(a) Wade, P. A.; Hinney, H. R. Benzenesulfonylnitrile Oxide: A Useful Intermediate for the syn-Cyanohydroxylation of Alkenes. J. Am. Chem. Soc. 1979, 101, 1319−1320; (b) Kozikowski, A. P.; Adamczyk, M. Methods for the Stereoselective cis Cyanohydroxylation and Carboxyhydroxylation of Olefins. J. Org. Chem. 1983, 48, 366–372; (c) Wade, P. A.; Bereznak, J. F. Sulfonylisoxazolines: Reliable Intermediates for the Preparation of β-Hydroxy Nitriles. J. Org. Chem. 1987, 52, 2973–2977; (d) You, Z.; Lee, H. J. One Step Conversion of Highly Dipolarophilic Olefins to α-Hydroxy-β-cyanoadducts with Metal Fulminate. Tetrahedron Lett. 1996, 37, 1165−1168; (e) Seo, M. H.; Lee, Y. Y.; Goo, Y. M. A new method for the preparation of β-hydroxy nitriles; Transformation of 3-bromo-2-isoxazolines to β-hydroxy nitriles by treatment of alkanethiolates. Synth. Commun. 1994, 24, 1433−1439; (f) Caetano, V. F.; Demnitz, F. W. J.; Diniz, F. B.; Mariz, R. M.; Navarro, M. Preparation of β-hydroxyesters from isoxazolines. A selective Ni0bpy-catalyzed electrochemical method. Tetrahedron Lett. 2003, 44, 8217−8220; (g) Kociolek, M. G.; Kalbarczyk, K. P. Ring Opening of 3-Bromo-2-Isoxazolines to β-Hydroxy Nitriles. Synth. Commun. 2004, 34, 4387−4394; (h) Singh, A.; Roth, G. P. A [3+2] Dipolar Cycloaddition Route to 3-Hydroxy-3-alkyl Oxindoles: An Approach to Pyrrolidinoindoline Alkaloids. Org. Lett. 2011, 13, 2118−2121; (i) Zheng, D.; Asano, Y. Biocatalytic asymmetric ring-opening of dihydroisoxazoles: a cyanide-free route to complementary enantiomers of β-hydroxy nitriles from olefins. Green Chem. 2020, 22, 4930−4936.
- 10 Kiyokawa, K.; Ishizuka, M.; Minakata, S. Stereospecific Oxycyanation of Alkenes with Sulfonyl Cyanide. Angew. Chem. Int. Ed. 2023, 62, e202218743.
- 11 Liu, H.; Wang, Y.-P.; Wang, H.; Ren, K.; Liu, L.; Dang, L.; Wang, C.-Q.; Feng, C. Photocatalytic Multisite Functionalization of Unactivated Terminal Alkenes by Merging Polar Cycloaddition and Radical Ring-Opening Process. Angew. Chem. Int. Ed. 2024, 63, e202407928.
- 12 Wanderley, T. A. S.; Buscemi, R.; Conboy, Ó.; Knight, B.; Crisenza, G. E. M. General Alkene 1,2-syn-Cyano-Hydroxylation Procedure via Electrochemical Activation of Isoxazoline Cycloadducts. J. Am. Chem. Soc. 2024, 146, 32848−32858.
- 13(a) Tian, P.; Wang, C.-Q.; Cai, S.-H.; Song, S.; Ye, L.; Feng, C.; Loh, T.-P. F− Nucleophilic-Addition-Induced Allylic Alkylation. J. Am. Chem. Soc. 2016, 138, 15869−15872; (b) Tang, H.-J.; Lin, L.-Z.; Feng, C.; Loh, T.-P. Palladium-Catalyzed Fluoroarylation of gem-Difluoroalkenes. Angew. Chem. Int. Ed. 2017, 56, 9872−9876; (c) Zhang, Y.; Liu, H.; Tang, L.; Tang, H.-J.; Wang, L.; Zhu, C.; Feng, C. Intermolecular Carboamination of Unactivated Alkenes. J. Am. Chem. Soc. 2018, 140, 10695−10699; (d) Liu, H.; Ge, L.; Wang, D.-X.; Chen, N.; Feng, C. Photoredox-Coupled F-Nucleophilic Addition: Allylation of gem Difluoroalkenes. Angew. Chem. Int. Ed. 2019, 58, 3918−3922; (e) Wang, L.; Zhang, H.; Zhu, C.; Feng, C. Expedient Trifluoromethylacylation of Styrenes Enabled by Photoredox Catalysis. Chin. J. Chem. 2022, 40, 59−64; (f) Chen, K.; Liu, Q.; Wan, J.; Zhu, C.; Feng, C. Ni-Catalyzed Reductive Dibenzylation of Trifluoromethylalkenes for CF3-Containing All-Carbon Quaternary Center Construction. Org. Lett. 2023, 25, 5995−6000; (g) Li, Z.-Q.; Tang, H.-J.; Wang, Z.; Wang, C.-Q.; Feng, C. Multi-substituted trifluoromethyl alkene construction via gold- catalyzed fluoroarylation of gem-difluoroallenes. Chem. Sci. 2024, 15, 3524−3529; (h) Wang, C.-Q.; Feng, C. Applications of Nucleophilic Fluorine Sources in the Selective Fluorofunctionalization of Unsaturated Carbon-Carbon Bonds. Acta Chim. Sinica 2024, 82, 160−170.
- 14(a) Juliá, F.; Constantin, T.; Leonori, D. Applications of Halogen-Atom Transfer (XAT) for the Generation of Carbon Radicals in Synthetic Photochemistry and Photocatalysis. Chem. Rev. 2022, 122, 2292–2352; (b) Sachidanandan, K.; Niu, B.; Laulhé, S. An Overview of α-Aminoalkyl Radical Mediated Halogen Atom Transfer. ChemCatChem 2023, 15, e202300860; (c) Jiang, Y.; Yin, Y.; Jiang, Z. Recent Advances in Strategies for Halide Atom Transfer (XAT) and Their Applications. Chin. J. Org. Chem. 2024, 44, 1733–1759.
- 15Selected examples, see: (a) Zhang, P.; Le, C. C.; MacMillan, D. W. C. Silyl Radical Activation of Alkyl Halides in Metallaphotoredox Catalysis: A Unique Pathway for Cross-Electrophile Coupling. J. Am. Chem. Soc. 2016, 138, 8084−8087; (b) ElMarrouni, A.; Ritts, C. B.; Balsells, J. Silyl-mediated photoredox-catalyzed Giese reaction: addition of non-activated alkyl bromides. Chem. Sci. 2018, 9, 6639−6646; (c) Bacauanu, V.; Cardinal, S.; Yamauchi, M.; Kondo, M.; Fernández, D. F.; Remy, R.; MacMillan, D. W. C. Metallaphotoredox Difluoromethylation of Aryl Bromides. Angew. Chem. Int. Ed. 2018, 57, 12543−12548.




