Skeleton Rearranged and Oxygenated ent-Rosane Diterpenoids with Antiadipogenic Activity from Euphorbia milii
Qin-Qin Song
Shandong Laboratory of Yantai Drug Discovery, Bohai Rim Advanced Research Institute for Drug Discovery, 198 East Binhai Road, Yantai, Shandong, 264117 China
Search for more papers by this authorYue Guo
Shandong Laboratory of Yantai Drug Discovery, Bohai Rim Advanced Research Institute for Drug Discovery, 198 East Binhai Road, Yantai, Shandong, 264117 China
University of Chinese Academy of Sciences, Beijing, 100049 China
State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, 555 Zuchongzhi Road, Shanghai, 201203 China
Search for more papers by this authorPeng Sun
CAS Key Laboratory of Tropical Plant Resources and Sustainable Use, Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences, No. 88, Xuefu Rd, Kunming, Yunnan, 650223 China
Search for more papers by this authorCorresponding Author
Yao-Yue Fan
Shandong Laboratory of Yantai Drug Discovery, Bohai Rim Advanced Research Institute for Drug Discovery, 198 East Binhai Road, Yantai, Shandong, 264117 China
University of Chinese Academy of Sciences, Beijing, 100049 China
State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, 555 Zuchongzhi Road, Shanghai, 201203 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Kai-Long Ji
CAS Key Laboratory of Tropical Plant Resources and Sustainable Use, Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences, No. 88, Xuefu Rd, Kunming, Yunnan, 650223 China
E-mail: [email protected]; [email protected]Search for more papers by this authorQin-Qin Song
Shandong Laboratory of Yantai Drug Discovery, Bohai Rim Advanced Research Institute for Drug Discovery, 198 East Binhai Road, Yantai, Shandong, 264117 China
Search for more papers by this authorYue Guo
Shandong Laboratory of Yantai Drug Discovery, Bohai Rim Advanced Research Institute for Drug Discovery, 198 East Binhai Road, Yantai, Shandong, 264117 China
University of Chinese Academy of Sciences, Beijing, 100049 China
State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, 555 Zuchongzhi Road, Shanghai, 201203 China
Search for more papers by this authorPeng Sun
CAS Key Laboratory of Tropical Plant Resources and Sustainable Use, Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences, No. 88, Xuefu Rd, Kunming, Yunnan, 650223 China
Search for more papers by this authorCorresponding Author
Yao-Yue Fan
Shandong Laboratory of Yantai Drug Discovery, Bohai Rim Advanced Research Institute for Drug Discovery, 198 East Binhai Road, Yantai, Shandong, 264117 China
University of Chinese Academy of Sciences, Beijing, 100049 China
State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, 555 Zuchongzhi Road, Shanghai, 201203 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Kai-Long Ji
CAS Key Laboratory of Tropical Plant Resources and Sustainable Use, Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences, No. 88, Xuefu Rd, Kunming, Yunnan, 650223 China
E-mail: [email protected]; [email protected]Search for more papers by this authorComprehensive Summary
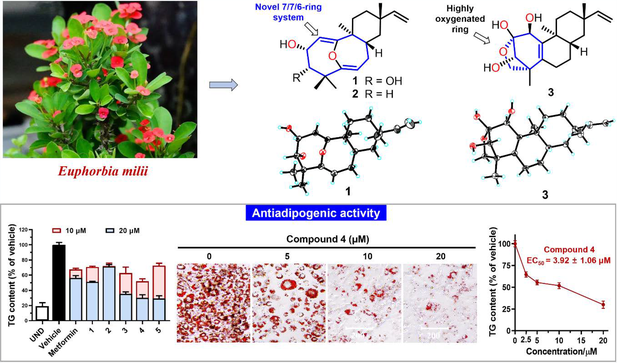
Five unreported and oxygenated ent-rosane diterpenoids (ent-RDs), Euphomillanols A—E (1—5), were isolated from Euphorbia milii. Among them, compounds 1 and 2 are unprecedented 7/7/6-fused tricyclic 5,10-seco-ent-RDs and possess a unique 11-oxabicyclo[4.4.1]undeca-1(10),5-diene moiety, while 3 is characterized by a 1-methyl-6-oxabicyclo[3.2.1]oct-2-ene motif of ring A. Their structures with absolute configurations were unambiguously determined by the extensive spectroscopic methods, X-ray crystallography, and ECD calculation. Putative biosynthetic pathways for compounds 1—3 are proposed. All compounds exhibited antiadipogenic effects in 3T3-L1 adipocytes, and the most potent compound 4 showed an EC50 value of 3.92 μmol/L with low cytotoxicity (IC50 > 89.54 μmol/L).
Supporting Information
| Filename | Description |
|---|---|
| cjoc202400749-sup-0001-supinfo.pdfPDF document, 7.3 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Health Service Delivery Framework for Prevention and Management of Obesity, World Health Organization, 2023, https://www.who.int/publications/i/item/9789240073234
- 2 Kopelman, P. G. Obesity as a medical problem. Nature 2000, 404, 635–643.
- 3 Van Gaal, L. F.; Rissanen, A. M.; Scheen, A. J.; Ziegler, O.; Rössner, S. Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet 2005, 365, 1389−1397.
- 4 James, W. P. T.; Caterson, I. D.; Coutinho, W.; Finer, N.; Van Gaal, L. F.; Maggioni, A. P.; Torp-Pedersen, C.; Sharma, A. M.; Shepherd, G. M.; Rode, R. A.; Renz, C. L. Effect of sibutramine on cardiovascular outcomes in overweight and obese subjects. N. Engl. J. Med. 2010, 363, 905−917.
- 5 Sharretts, J.; Galescu, O.; Gomatam, S.; Andraca-Carrera, E.; Hampp, C.; Yanoff, L. Cancer risk associated with lorcaserin-the FDA's review of the CAMELLIA-TIMI 61 trial. N. Engl. J. Med. 2020, 383, 1000–1002.
- 6 Wen, X.; Zhang, B. H.; Wu, B. Y.; Xiao, H. T.; Li, Z. H.; Li, R. Y.; Xu, X. W.; Li, T. Signaling pathways in obesity: mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2022, 7, 298.
- 7 Fan, Q. J.; Xu, F. R.; Liang, B.; Zou, X. J. The anti-obesity effect of traditional Chinese medicine on lipid metabolism. Front. Pharmacol. 2021, 12, 696603.
- 8 Zhao, J. X.; Yue, J. M. Frontier studies on natural products: moving toward paradigm shifts. Sci. China Chem. 2023, 66, 928−942.
- 9 Liu, J. L.; Lee, J.; Hernandez, M. A. S.; Mazitschek, R.; Ozcan, U. Treatment of obesity with celastrol. Cell 2015, 161, 999−1011.
- 10 Dai, J. Y.; Liang, K.; Zhao, S.; Jia, W. T.; Liu, Y.; Wu, H. K.; Lv, J.; Cao, C.; Chen, T.; Zhuang, S. T.; Hou, X. M.; Zhou, S. J.; Zhang, X. N.; Chen, X. W.; Huang, Y. Y.; Xiao, R. P.; Wang, Y. L.; Luo, T. P.; Xiao, J. Y.; Wang, C. Chemoproteomics reveals baicalin activates hepatic CPT1 to ameliorate diet-induced obesity and hepatic steatosis. Proc. Natl. Acad. Sci. U. S. A. 2018, 115, E5896–E5905.
- 11 Rao, Y.; Yu, H.; Gao, L.; Lu, Y. T.; Xu, Z., Liu, H.; Gu, L. Q.; Ye, J. M.; Huang, Z. S. Natural alkaloid bouchardatine ameliorates metabolic disorders in high-fat diet-fed mice by stimulating the sirtuin 1/liver kinase B-1/AMPK axis. Brit. J. Pharmacol. 2017, 174, 2457−2470.
- 12 Song, Q. Q.; Rao, Y.; Tang, G. H.; Sun, Z. H.; Zhang, J. S.; Huang, Z. S.; Yin, S. Tigliane diterpenoids as a new type of antiadipogenic agents inhibit GRα-Dexras1 axis in adipocytes. J. Med. Chem. 2019, 62, 2060−2075.
- 13 Yan, X. L.; Pan, Y. H.; Fan, R. Z.; Song, Q. Q.; Li, S.; Huang, J. L.; Li, W.; Huang, D.; Yuan, F. Y.; Tang, G. H.; Rao, Y.; Huang, Z. S.; Yin, S. Discovery of the first raptor (regulatory-associated protein of mTOR) inhibitor as a new type of antiadipogenic agent. J. Med. Chem. 2023, 66, 5839−5858.
- 14 Zhao, H.; Sun, L.; Kong, C. H.; Mei, W. L.; Dai, H. F.; Xu, F. Q.; Huang, S. Z. Phytochemical and pharmacological review of diterpenoids from the genus Euphorbia Linn (2012-2021). J. Ethnopharmacol. 2022, 298, 115574.
- 15 Zhan, Z. J.; Li, S.; Chu, W.; Yin, S. Euphorbia diterpenoids: isolation, structure, bioactivity, biosynthesis, and synthesis (2013–2021). Nat. Prod. Rep. 2022, 39, 2132–2174.
- 16 Zhao, Y.; Hua, C.; Sha, Y. O.; Wu, P. Q.; Liu, Q. F.; Lu, L.; Zhou, B.; Jiang, S. B.; Fan, Y.-Y.; Yue, J. M. Diterpenoids from Euphorbia lactea and their anti-HIV-1 activity. Phytochemistry 2023, 213, 113745.
- 17 Zhang, Z. Y.; Li, Y.; Yu, J. H.; Zhao, J. X.; Yue, J. M. Lauinoids A–X: labdane-type diterpenoids with anti-inflammatory activity from Croton laui. Phytochemistry 2024, 223, 114138.
- 18 Flora of China Editorial Committee. Flora Reipublicae Popularis Sinicae, Vol. 44, Issue 3, Science Press, Beijing, 1994, pp. 58–60.
- 19 Liu, S. N.; Huang, D.; Morris-Natschke, S. L.; Ma, H.; Liu, Z. H.; Seeram, N. P.; Xu, J.; Lee, K. H.; Gu, Q. Euphomilones A and B, ent-rosane diterpenoids with 7/5/6 and 5/7/6 skeletons from Euphorbia milii. Org. Lett. 2016, 18, 6132–6135.
- 20 Liu, S. N.; Hu, J. Y.; Tan, S. H.; Wang, Q.; Xu, J.; Wang, Y.; Yuan, Y.; Gu, Q. ent-Rosane diterpenoids from Euphorbia milii showing an Epstein-Barr virus lytic replication assay. RSC Adv. 2017, 7, 46938–46947.
- 21 Yu, H. F.; Cheng, Y. C.; Wu, C. M.; Ran, K.; Wei, B.; Xu, Y. K.; Shan, W. G.; Ying, Y. M.; Zhan, Z. J. Diverse diterpenoids with α-glucosidase and β-glucuronidase inhibitory activities from Euphorbia milii. Phytochemistry 2022, 196, 113106.
- 22 Peng, X.; Liu, S. N.; Zhang, Y. T.; Xu, J.; Gu, Q. Identification and structural modification of ent-rosane diterpenoids from Euphorbia milii inhibiting RANKL-induced osteoclastogenesis. Bioorg. Chem. 2024, 145, 107253.
- 23 Ji, K. L.; Fan, Y. Y.; Kuok, H. H.; Liu, Q. F.; Li, T.; Yue, J. M. Macrocyclic nonapeptides incorporating uncharacterized amino acids with inhibitory effects on Th17 differentiation. CCS Chem. 2021, 3, 844–858.
- 24 Wu, M. Z.; GongPan, P. C.; Dai, M. Y.; Sun, P.; Huang, T. P.; Xu, Y. K.; Xiao, C. F.; Li, J.; Sun, Y. L.; Ji, K. L. Dimeric styrylpyrones with stimulating GLP-1 secretion activities from Alpinia kwangsiensis. Tetrahedron 2022, 120, 132901.
- 25 Fan, Y. Y.; Zheng, C. Y.; Zhou, B.; Zimbres, F. M.; Cassera, M. B.; Yue, J. M. Two terpenoid derivatives with different 3/5/6/6/5-fused pentacyclic skeletons from Hedyosmum orientale. Chin. J. Chem. 2023, 41, 392–398.
- 26 Ji, K. L.; Wu, M. Z.; Huang, C. Y.; GongPan, P. C.; Sun, P.; Sun, Y. L.; Li, J.; Xiao, C. F.; Xu, Y. K.; Fan, Q. F.; Hu, H. B.; Song, Q. S. Alpinia hainanensis rhizome extract ameliorates dextran sulfate sodium-induced ulcerative colitis: active ingredient investigation and evaluation. J. Agric. Food Chem. 2022, 70, 3989−3999.
- 27 Gao, X. H.; Wu, P. Q.; Fan, Y. Y.; Zhou, B.; Yue, J. M. Suadimins D-J, monoterpenoid indole-quinoline and bisindole alkaloids from Melodinus suaveolens. Chin. J. Chem. 2023, 41, 2296–2304.
- 28 Ji, K. L.; Tang, Y. Q.; Guo, Z. Y. GongPan, P. C.; Sun, P.; Lu, J. M.; Dai, M. Y.; Li, T. Natural geranylated sulfur-containing amides with inhibitory effect on Th17 differentiation. Chem. Biodivers. 2023, 20, e202300373.
- 29 Zhou, J. S.; Cheng, L.; Gao, Y.; Ge, Z. P.; Zhou, B.; Li, J. Y.; Zhao, J. X.; Yue, J. M. Highly aromatic norditerpenoid heterodimers and monomers from Trigonostemon fragilis. Engineering 2024, 38, 144–154.
- 30 Ji, K. L.; Liu, W.; Yin, W. H.; Kong, X. R.; Xu, H. H.; Lai, Z. W.; Li, J. Y.; Yue, J. M. A new class of potent liver injury protective compounds: Structural elucidation, total synthesis and bioactivity study. Acta Pharm. Sin. B 2023, 13, 3414−3424.
- 31 Ji, K. L.; Fan, Y. Y.; Gong, Q.; Liu, Q. F.; Cui, M. J.; Fu, K. C.; Zhang, H. Y.; Yue, J. M. Densely functionalized macrocyclic sesquiterpene pyridine alkaloids from Maytenus austroyunnanensis. J. Nat. Prod. 2023, 86, 2315−2325.
- 32 Frelek, J.; Ikekawa, N.; Takatsuto, S.; Snatzke, G. Application of [Mo2(OAc)4] for determination of absolute configuration of brassinosteroid vic-diols by circular dichroism. Chirality 1997, 9, 578−582.
- 33 Di Bari, L.; Pescitelli, G., Pratelli, C.; Pini, D.; Salvadori, P. Determination of absolute configuration of acyclic 1,2-diols with Mo2(OAc)4. 1. Snatzke's method revisited. J. Org. Chem. 2001, 66, 4819−4825.
- 34 Zheng, G. J.; Jin, P. F.; Huang, L.; Zhang, Q. H.; Meng, L. K.; Yao, G. M. Structurally diverse diterpenoids from Pieris japonica as potent analgesics. Bioorg. Chem. 2020, 99, 103794.
- 35 Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. A.; Nakatsuji, H.; Caricato, M.; Li, X.; Hratchian, H. P.; Izmaylov, A. F.; Bloino, J.; Zheng, G.; Sonnenberg, J. L.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Montgomery, Jr., J. A.; Peralta, J. E.; Ogliaro, F.; Bearpark, M.; Heyd, J. J.; Brothers, E.; Kudin, K. N.; Staroverov, V. N.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A.; Burant, J. C.; Iyengar, S. S.; Tomasi, J.; Cossi, M.; Rega, N.; Millam, J. M.; Klene, M.; Knox, J. E.; Cross, J. B.; Bakken, V.; Adamo, C.; Jaramillo, J.; Gomperts, R.; Stratmann, R. E.; Yazyev, O.; Austin, A. J.; Cammi, R.; Pomelli, C.; Ochterski, J. W.; Martin, R. L.; Morokuma, K.; Zakrzewski, V. G.; Voth, G. A.; Salvador, P.; Dannenberg, J. J.; Dapprich, S.; Daniels, A. D.; Farkas, Ö.; Foresman, J. B.; Ortiz, J. V.; Cioslowski, J.; Fox, D. J. Gaussian 09, Revision A.1, Gaussian, Inc., Wallingford CT, 2009.
- 36 Bruhn, T.; Schaumlöffel, A.; Hemberger, Y.; Bringmann, G. SpecDis: quantifying the comparison of calculated and experimental electronic circular dichroism spectra. Chirality 2013, 25, 243−249.
- 37 Pescitelli, G.; Bruhn, T. Good computational practice in the assignment of absolute configurations by TDDFT calculations of ECD spectra. Chirality 2016, 28, 466−474.
- 38 Bruhn, T.; Schaumlöffel, A.; Hemberger, Y.; Pescitelli, G. SpecDis version1.71, Berlin, Germany, 2017.




