Oxidative Alkylarylation of N-Aryl Bicyclobutyl Amides with C(sp3)–H Feedstocks via C(sp3)–H/C(sp2)–H Functionalization
Jing Yuan
Department of Chemistry and Chemical Engineering, Hunan Institute of Science and Technology, Yueyang, Hunan, 414006 China
Search for more papers by this authorJiao Zhou
Department of Chemistry and Chemical Engineering, Hunan Institute of Science and Technology, Yueyang, Hunan, 414006 China
Search for more papers by this authorPeng-Fei Xia
Department of Chemistry and Chemical Engineering, Hunan Institute of Science and Technology, Yueyang, Hunan, 414006 China
Search for more papers by this authorYu Liu
Department of Chemistry and Chemical Engineering, Hunan Institute of Science and Technology, Yueyang, Hunan, 414006 China
Search for more papers by this authorKe-Wen Tang
Department of Chemistry and Chemical Engineering, Hunan Institute of Science and Technology, Yueyang, Hunan, 414006 China
Search for more papers by this authorCorresponding Author
Jian-Hong Fan
Department of Chemistry and Chemical Engineering, Hunan Institute of Science and Technology, Yueyang, Hunan, 414006 China
E-mail: [email protected]Search for more papers by this authorJing Yuan
Department of Chemistry and Chemical Engineering, Hunan Institute of Science and Technology, Yueyang, Hunan, 414006 China
Search for more papers by this authorJiao Zhou
Department of Chemistry and Chemical Engineering, Hunan Institute of Science and Technology, Yueyang, Hunan, 414006 China
Search for more papers by this authorPeng-Fei Xia
Department of Chemistry and Chemical Engineering, Hunan Institute of Science and Technology, Yueyang, Hunan, 414006 China
Search for more papers by this authorYu Liu
Department of Chemistry and Chemical Engineering, Hunan Institute of Science and Technology, Yueyang, Hunan, 414006 China
Search for more papers by this authorKe-Wen Tang
Department of Chemistry and Chemical Engineering, Hunan Institute of Science and Technology, Yueyang, Hunan, 414006 China
Search for more papers by this authorCorresponding Author
Jian-Hong Fan
Department of Chemistry and Chemical Engineering, Hunan Institute of Science and Technology, Yueyang, Hunan, 414006 China
E-mail: [email protected]Search for more papers by this authorComprehensive Summary
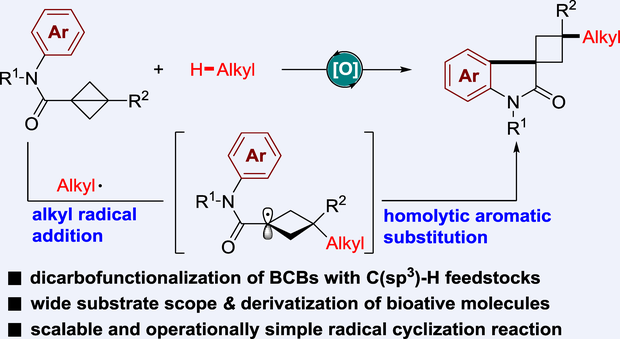
The difunctionalization of bicyclo[1.1.0]butanes is an under-explored transformation that accesses to moieties that are otherwise difficult to prepare. Herein, a new oxidative radical alkylarylation of N-aryl bicyclobutyl amides with C(sp3)−H feedstocks is achieved in an atom-economic and photocatalyst- and light-free manner. This protocol follows a sequential C(sp3)–H/C(sp2)–H functionalization, providing an efficient route for diversity-oriented synthesis of functionalized 3-spirocyclobutyl oxindoles. In particular, a wide range of C(sp3)−H feedstocks, including ether, alcohol, amine, thioether, polychlorinated methane, silane, acetone, acetonitrile, toluene, and alkane are all suitable for the C(sp3)−H functionalization, demonstrating the broad applicability of this transformation.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202400748-sup-0001-supinfo.pdfPDF document, 9 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Turkowska, J.; Durka, J.; Gryko, D. Strain Release – an Old Tool for New Transformations. Chem. Commun. 2020, 56, 5718–5734; (b) Fawcett, A. Recent Advances in the Chemistry of Bicyclo- and 1-Azabicyclo[1.1.0]butanes. Pure Appl. Chem. 2020, 92, 751–765; (c) Bellotti, P.; Glorius, F. Strain-Release Photocatalysis. J. Am. Chem. Soc. 2023, 145, 20716–20732.
- 2(a) Walczak, M. A. A.; Krainz, T.; Wipf, P. Ring-Strain-Enabled Reac-tion Discovery: New Heterocycles from Bicyclo[1.1.0]butanes. Acc. Chem. Res. 2015, 48, 1149–1158; (b) Kelley, C. B.; Milligan, J. A.; Tilley, L. J.; Sodano, T. M. Bicyclobutanes: from Curiosities to Versatile Reagents and Covalent Warheads. Chem. Sci. 2022, 13, 11721–11737; (c) Golfmann, M.; Walker, J. C. L. Bicyclobutanes as Unusual Building Blocks for Complexity Generation in Organic Synthesis. Commun. Chem. 2023, 6, 9.
- 3(a) Panish, R.; Chintala, S. R.; Boruta, D. T.; Fang, Y.; Taylor, M. T.; Fox, J. M. Enantioselective Synthesis of Cyclobutanes via Sequential Rh-catalyzed Bicyclobutanation/Cu-catalyzed Homoconjugate Addition. J. Am. Chem. Soc. 2013, 135, 9283–9286; (b) Milligan, J. A.; Busacca, C. A.; Senanayake, C. H.; Wipf, P. Hydrophosphination of Bicyclo[1.1.0]butane-1-carbonitriles. Org. Lett. 2016, 18, 4300–4303; (c) Gianatassio, R.; Lopchuk, J. M.; Wang, J.; Pan, C.-M.; Malins, L. R.; Prieto, L.; Brandt, T. A.; Collins, M. R.; Gallego, G. M.; Sach, N. W.; Spangler, J. E.; Zhu, H.; Zhu, J.; Baran, P. S. Strain-release Amination. Science 2016, 351, 241–246; (d) Lopchuk, J. M.; Fjelbye, K.; Kawamata, Y.; Malins, L. R.; Pan, C.-M.; Gianatassio, R.; Wang, J.; Prieto, L.; Bradow, J.; Brandt, T. A.; Collins, M. R.; Elleraas, J.; Ewanicki, J.; Farrell, W.; Fadeyi, O. O.; Gallego, G. M.; Mousseau, J. J.; Oliver, R.; Sach, N. W.; Smith, J. K.; Spangler, J. E.; Zhu, H.; Zhu, J.; Baran, P. S. Strain-Release Heteroatom Functionalization: Development, Scope, and Stereospecificity. J. Am. Chem. Soc. 2017, 139, 3209–3226; (e) Zhang, P.; Zhuang, R.; Wang, X.; Liu, H.; Li, J.; Su, X.; Chen, X.; Zhang, X. Highly Efficient and Stable Strain-Release Radioiodination for Thiol Chemoselective Bioconjugation. Bioconjugate Chem. 2018, 29, 467–472; (f) Tokunaga, K., Sato, M.; Kuwata, K.; Miura, C.; Fuchida, H.; Matsunaga, N.; Koyanagi, S.; Ohdo, S.; Shindo, N.; Ojida, A. Bicyclobutane Carboxylic Amide as a Cysteine-Directed Strained Electrophile for Selective Targeting of Proteins. J. Am. Chem. Soc. 2020, 142, 18522–18531; (g) Schwartz, B. D.; Zhang, M. Y.; Attard, R. H.; Gardiner, M. G.; Malins, L. R. Structurally Diverse Acyl Bicyclobutanes: Valuable Strained Electrophiles. Chem. Eur. J. 2020, 26, 2808–2812; (h) Guin, A.; Bhattacharjee, S.; Harariy, M. S.; Biju. A. T. Lewis Acid-Catalyzed Diastereoselective Carbofunctionalization of Bicyclobutanes Employing Naphthols. Chem. Sci. 2023, 14, 6585–6591; (i) Tang, L.; Huang, Q.-N.; Wu, F.; Xiao, Y.; Zhou, J.-L.; Xu, T.-T.; Wu, W.-B.; Qu, S.; Feng, J. C(sp2)–H Cyclobutylation of Hydroxyarenes Enabled by Silver-π-acid Catalysis: Diastereocontrolled Synthesis of 1,3-Difunctionalized Cyclobutanes. Chem. Sci. 2023, 14, 9696–9703.
- 4(a) Wu, X.; Hao, W.; Ye, K.-Y.; Jiang, B.; Pombar, G.; Song, Z.; Lin, S. Ti-Catalyzed Radical Alkylation of Secondary and Tertiary Alkyl Chlorides Using Michael Acceptors. J. Am. Chem. Soc. 2018, 140, 14836–14843; (b) Ernouf, G.; Chirkin, E.; Rhyman, L.; Ramasami, P.; Cintrat, J.-C. Photochemical Strain-Release-Driven Cyclobutylation of C(sp3)-Centered Radicals. Angew. Chem. Int. Ed. 2020, 59, 2618–2622; (c) Pratt, C. J.; Ayock, R. A.; King, M. D.; Jui, N. T. Radical α-C-H Cyclobutylation of Aniline Derivatives. Synlett 2020, 31, 51–54; (d) Yu, X.; Lübbesmeyer, M.; Studer, A. Oligosilanes as Silyl Radical Precursors through Oxidative Si-Si Bond Cleavage Using Redox Catalysis. Angew. Chem. Int. Ed. 2021, 60, 675–679; (e) Gao, H.; Guo, L.; Shi, C.-C.; Zhu, Y.-N.; Yang, C.; Xia, W.-J. Transition Metal-Free Radical α-Oxy Cyclobutylation via Photoinduced Hydrogen Atom Transfer. Adv. Synth. Catal. 2022, 364, 2140–2145; (f) Chen, P.-F.; Li, D.-S.; Ou, W.-T.; Xue, F.; Deng, H.-P. 2-Isopropylthioxanthone-Catalyzed Divergent Functionalization of Bicyclo[1.1.0]butanes under Visible-Light Irradiation. Org. Lett. 2023, 25, 6184–6188.
- 5(a) Ociepa, M.; Wierzba, A. J.; Turkowska, J.; Gryko, D. Polarity-Reversal Strategy for Functionalization of Electrophilic Strained Molecules via Light-Driven Cobalt Catalysis. J. Am. Chem. Soc. 2020, 142, 5355–5361; (b) Zhang, Z.; Gevorgyan, V. Palladium Hydride-Enabled Hydroalkenylation of Strained Molecules. J. Am. Chem. Soc. 2022, 144, 20875–20883.
- 6(a) Li, M.; Song, R.-J.; Li, J.-H. Nickel-Promoted Oxidative ipso-Annulation of N-(p-Methoxyaryl)propiolamides with α-Carbonyl Alkyl Bromides. Chin. J. Chem. 2017, 35, 299–302; (b) Zeng, L.; Qin, J.-H.; Lv, G.-F.; Hu, M.; Sun, Q.; Ouyang, X.-H.; He, D.-L.; Li, J.-H. Electrophotocatalytic Reductive 1,2-Diarylation of Alkenes with Aryl Halides and Cyanoaromatics. Chin. J. Chem. 2023, 41, 1921–1930.
- 7(a) Pickford, H. D.; Ripenko, V.; McNamee, R. E.; Holovchuk, S.; Thompson, A. L.; Smith, R. C.; Mykhailiuk, P. K.; Anderson, E. A. Rapid and Scalable Halosulfonylation of Strain-Release Reagents. Angew. Chem. Int. Ed. 2023, 62, e202213508; (b) Duan, Y.; Xu, Y.; Li, Y.; Mao, L.; Feng, J.; Zhang, R.; Tang, W.; Lu, T.; Chen, Y.; Feng, J. Photochemical Selective Difluoroalkylation Reactions of Bicyclobutanes: Direct Sustainable Pathways to Functionalized Bioisosteres for Drug Discovery. Green Chem. 2024, 26, 5512–5518; (c) Wang, H.; Erchinger, J. E.; Lenz, M.; Dutta, S.; Daniliuc, C. G.; Glorius, F. syn-Selective Difunctionalization of Bicyclobutanes Enabled by Photoredox-Mediated C−S σ-Bond Scission. J. Am. Chem. Soc. 2023, 145, 23771–23780; (d) Kraemer, Y.; Buldt, J. A.; Kong, W.-Y.; Stephens, A. M.; Ragan, A. N.; Park, S.; Haidar, Z. C.; Patel, A. H.; Shey, R.; Dagan, R.; McLoughlin, C. P.; Fettinger, J. C.; Tantillo, D. J.; Pitts, C. R. Overcoming a Radical Polarity Mismatch in Strain-Release Pentafluorosulfanylation of [1.1.0]Bicyclobutanes: An Entryway to Sulfone- and Carbonyl-Containing SF5-Cyclobutanes. Angew. Chem. Int. Ed. 2024, 63, e202319930.
- 8(a) Hartwig, J. F.; Larsen, M. A. Undirected, Homogeneous C−H Bond Functionalization: Challenges and Opportunities. ACS Central Sci. 2016, 2, 281–292; (b) Cao, H.; Tang, X.; Tang, H.; Yuan, Y.; Wu, J. Photoinduced Intermolecular Hydrogen Atom Transfer Reactions in Organic synthesis. Chem. Catal. 2021, 1, 523–598; (c) Li, J.; Huang, C.-Y.; Li, C.-J. Cross-dehydrogenative Coupling of Unactivated Alkanes. Trends Chem. 2022, 4, 479–494; (d) Golden, D. L.; Suh, S.-E.; Stahl, S. S. Radical C(sp3)–H Functionalization and Cross-Coupling Reactions. Nat. Rev. Chem. 2022, 6, 405–427; (e) Zhang, Z.; Chen, P.; Liu, G. Copper-catalyzed Radical Relay in C(sp3)−H Functionalization. Chem. Soc. Rev. 2022, 51, 1640–1658; (f) Galeotti, M.; Salamone, M.; Bietti, M. Electronic Control over Site-selectivity in Hydrogen Atom Transfer (HAT) based C(sp3)−H Functionalization Promoted by Electrophilic Reagents. Chem. Soc. Rev. 2022, 51, 2171–2223; (g) Wei, W.-T.; Zhou, M.-B.; Fan, J.-H.; Liu, W.; Song, R.-J.; Liu, Y.; Hu, M.; Xie, P.; Li, J.-H. Synthesis of Oxindoles by Iron-Catalyzed Oxidative 1,2-Alkylarylation of Activated Alkenes with an Aryl C(sp2)–H Bond and a C(sp3)–H Bond Adjacent to a Heteroatom. Angew. Chem. Int. Ed. 2013, 52, 3638–3641.
- 9 Fan, J.-H.; Yuan, J.; Xia, P.-F.; Zhou, J.; Zhong, L.-J.; Huang, P.-F.; Liu, Y.; Tang, K.-W.; Li, J.-H. Photoredox-Catalyzed Alkylarylation of N-Aryl Bicyclobutyl Amides with α-Carbonyl Alkyl Bromides: Access to 3-Spirocyclobutyl Oxindoles. Org. Lett. 2024, 26, 2073–2078.
- 10(a) Hell, S. M.; Meyer, C. F.; Laudadio, G.; Misale, A.; Willis, M. C.; Noël, T.; Trabanco, A. A.; Gouverneur, V. Silyl Radical-Mediated Activation of Sulfamoyl Chlorides Enables Direct Access to Aliphatic Sulfonamides from Alkenes. J. Am. Chem. Soc. 2020, 142, 720–725; (b) Shi, S.-X.; Zhang, H.-H.; Wang, Y.-L.; Jiang, L.-H.; Xu, P.-F.; Luo, Y.-C. Visible-Light-Mediated Intermolecular [2+2]-Cycloaddition Reaction of 3-Alkylideneindolin-2-one with Alkenes via Triplet Energy Transfer for the Synthesis of 3-Spirocyclobutyl Oxindoles. Org. Lett. 2023, 25, 5426–5430.




