Divergent Assembly of Functionalized Pyrazolo[1,5-a]pyridine Derivatives via [3+2] Cyclization of N-Aminopyridinium Salts with Various Unsaturated Hydrocarbons
Xiang Liu
School of Chemistry and Chemical Engineering, Guangdong Pharmaceutical University, Zhongshan, Guangdong, 528458 China
Search for more papers by this authorShaohong Ma
School of Chemistry and Chemical Engineering, Guangdong Pharmaceutical University, Zhongshan, Guangdong, 528458 China
Search for more papers by this authorShi Yan
School of Chemistry and Chemical Engineering, Guangdong Pharmaceutical University, Zhongshan, Guangdong, 528458 China
Search for more papers by this authorXiaotian Shi
School of Chemistry and Chemical Engineering, Guangdong Pharmaceutical University, Zhongshan, Guangdong, 528458 China
Search for more papers by this authorShuting Li
School of Chemistry and Chemical Engineering, Guangdong Pharmaceutical University, Zhongshan, Guangdong, 528458 China
Search for more papers by this authorYanlong Ma
School of Chemistry and Chemical Engineering, Guangdong Pharmaceutical University, Zhongshan, Guangdong, 528458 China
Search for more papers by this authorCorresponding Author
Hua Cao
School of Chemistry and Chemical Engineering, Guangdong Pharmaceutical University, Zhongshan, Guangdong, 528458 China
E-mail: [email protected]Search for more papers by this authorXiang Liu
School of Chemistry and Chemical Engineering, Guangdong Pharmaceutical University, Zhongshan, Guangdong, 528458 China
Search for more papers by this authorShaohong Ma
School of Chemistry and Chemical Engineering, Guangdong Pharmaceutical University, Zhongshan, Guangdong, 528458 China
Search for more papers by this authorShi Yan
School of Chemistry and Chemical Engineering, Guangdong Pharmaceutical University, Zhongshan, Guangdong, 528458 China
Search for more papers by this authorXiaotian Shi
School of Chemistry and Chemical Engineering, Guangdong Pharmaceutical University, Zhongshan, Guangdong, 528458 China
Search for more papers by this authorShuting Li
School of Chemistry and Chemical Engineering, Guangdong Pharmaceutical University, Zhongshan, Guangdong, 528458 China
Search for more papers by this authorYanlong Ma
School of Chemistry and Chemical Engineering, Guangdong Pharmaceutical University, Zhongshan, Guangdong, 528458 China
Search for more papers by this authorCorresponding Author
Hua Cao
School of Chemistry and Chemical Engineering, Guangdong Pharmaceutical University, Zhongshan, Guangdong, 528458 China
E-mail: [email protected]Search for more papers by this authorComprehensive Summary
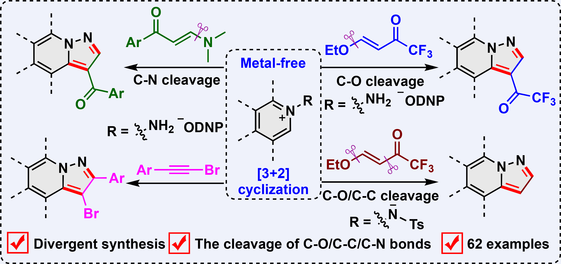
Two reaction modes for metal-free [3 + 2] cyclization of N-aminopyridinium derivatives with β-alkoxyvinyl trifluoromethylketones have been described through selective C—O or C—O/C—C bond cleavage. This strategy can also be extended to the [3 + 2] cyclization of N-aminopyridinium derivatives with enaminones and bromoalkynes. A broad range of N-aminopyridinium, N-aminoquinolinium, and N-aminoisoquinolinium salts are well tolerated, enabling the divergent synthesis of trifluoroacylated, non-substituted, acylated, and brominated pyrazolo[1,5-a]pyridine derivatives (62 examples).
Supporting Information
| Filename | Description |
|---|---|
| cjoc202400741-sup-0001-supinfo.pdfPDF document, 9.2 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Kim, M.; Koo, Y.; Hong, S. N-Functionalized Pyridinium Salts: A New Chapter for Site-Selective Pyridine C−H Functionalization via Radical-Based Processes under Visible Light Irradiation. Acc. Chem. Res. 2022, 55, 3043–3056; (b) Roychowdhury, P.; Samanta, S.; Tan, H.; Powers, D. C. N-Amino pyridinium salts in organic synthesis. Org. Chem. Front. 2023, 10, 2563–2580; (c) Pan, C.; Luo, S.; Wu, Y.; Yu, J.; Zhu, C. N-Amino pyridinium salts in organic synthesis. Org. Chem. Front. 2023, 10, 3479; (d) He, F.; Ye, S.; Wu, J. Recent Advances in Pyridinium Salts as Radical Reservoirs in Organic Synthesis. ACS Catal. 2019, 9, 8943–8960; (e) Wang, Y.; Bao, Y.; Tang, M.; Ye, Z.; Yuan, Z.; Zhu, G. Recent advances in difunctionalization of alkenes using pyridinium salts as radical precursors. Chem. Commun. 2022, 58, 3847–3864.
- 2(a) Kim, I.; Kang, G.; Lee, K.; Park, B.; Kang, D.; Jung, H.; He, Y.-T.; Baik, M.-H.; Hong, S. Site-Selective Functionalization of Pyridinium Derivatives via Visible-Light-Driven Photocatalysis with Quinolinone. J. Am. Chem. Soc. 2019, 141, 9239−9248; (b) Moon, Y.; Lee, W.; Hong, S. Visible-Light-Enabled Ortho-Selective Aminopyridylation of Alkenes with N-Aminopyridinium Ylides. J. Am. Chem. Soc. 2020, 142, 12420–12429; (c) Kim, J.; Kim, M.; Jeong, J.; Hong, S. Unlocking the Potential of β-Fragmentation of Aminophosphoranyl Radicals for Sulfonyl Radical Reactions. J. Am. Chem. Soc. 2023, 145, 14510–14518.
- 3(a) Tan, H.; Samanta, S.; Maity, A.; Roychowdhury, P.; Powers, D. C. N-Aminopyridinium reagents as traceless activating groups in the synthesis of N-Aryl aziridines. Nat. Commun. 2022, 13, 3341; (b) Samanta, S.; Biswas, P.; O'Bannon, B.; Powers, D. C. ß-Phenethylamine Synthesis via Latent Dual Electrophilicity of N-Pyridinium Aziridines. Angew. Chem. Int. Ed. 2024, 63, e202406335; (c) Shi, C.; Guo, L.; Gao, H.; Luo, M.; Yang, C.; Xia, W. Highly Diastereoselective Synthesis of γ-Lactams Enabled by Photoinduced Deaminative [3 + 2] Annulation Reaction. Org. Lett. 2022, 24, 4365–4370; (d) Shi, C.; Guo, L.; Gao, H.; Luo, M.; Zhou, X.; Yang, C.; Xia, W. Three-Component Aminoheteroarylation of Alkenes via Photoinduced EDA Complex Activation. Org. Lett. 2023, 25, 7661–7666; (e) He, M.; Shi, C.; Luo, M.; Yang, C.; Guo, L.; Zhao, Y.; Xia, W. Visible-Light-Driven Multicomponent Diamination and Oxyamination of Alkene. J. Org. Chem. 2024, 89, 1967–1979.
- 4(a) Saxena, B.; Patel, R. I.; Sharma, A. Recent Advances in Electron Donor-Acceptor (EDA)-Complex Reactions involving Quaternary Pyridinium Derivatives. Adv. Synth. Catal. 2023, 365, 1538–1564; (b) Pan, C.; Yang, Z.; Wu, X.; Yu, J.; Zhu, C. Substituent-Controlled Regioselective Photoinduced Cyclization of N-Allylbenzamides with N-Sulfonylaminopyridinium Salts. Org. Lett. 2023, 25, 494–499; (c) Cao, W.; Zhang, J.; Xu, M.; Liu, H.; Li, H.; Xu, X.; Ji, S. syn-Stereoselective C3-Spirocyclization and C2-Amination of 3-(2-Isocyanoethyl)indole Using C,N-Cyclic Azomethine Imines. Org. Lett. 2022, 24, 4620–4624; (d) Wang, P.; Liang, H.; Liu, W.; Zhou, L.-P.; Yu, W. Visible-Light- Driven [3 + 2]/[4 + 2] Annulation Reactions of Alkenes with N-Aminopyridinium Salts. Org. Lett. 2022, 24, 6037–6042; (e) Greulich, T. W.; Daniliuc, C. G.; Studer, A. N-Aminopyridinium Salts as Precursors for N-Centered Radicals-Direct Amidation of Arenes and Heteroarenes. Org. Lett. 2015, 17, 254–257.
- 5(a) Xiong, L.; Chen, F.; Wu, Y.; Hu, X.; Ruan, Z.; Jiang, H.; Zeng, W. Cyclocarboamination of Alkynes with N-Aminopyridiniums by Photoredox Catalysis. Org. Lett. 2022, 24, 7856–7860; (b) Zhao, P.; Liu, Y.; Zhang, Y.; Wang, L.; Ma, Y. Photodriven Radical-Polar Crossover Cyclization Strategy: Synthesis of Pyrazolo[1,5-a]pyridines from Diazo Compounds. Org. Lett. 2024, 26, 2511−2516; (c) Yu, W.; Wang, L.; Duan, Z. Bisphosphonium salt catalyzed [3 + 2] annulation of N-tosylimino(iso)quinolinium ylides with aryl olefins under blue LED irradiation. 2024, 11, 3827–3832.
- 6(a) Li, C.; Wang, C.-S.; Li, T.-Z.; Mei, G.-J.; Shi, F. Brønsted Acid- Catalyzed (4 + 3) Cyclization of N,N’-Cyclic Azomethine Imines with Isatoic Anhydrides. Org. Lett. 2019, 21, 598–602; (b) Wang, Z.; Li, X.; Qiu, J.; Li, W.; Li, H.; Weng, Z.; Li, H. Modular Access to 2-(Trifluoromethyl) pyrazolo[1,5-a] pyridines and Their Benzo Analogues through a Copper(I)-Catalyzed Radical Annulation. Org. Lett. 2022, 24, 6292–6297; (c) Wang, A.; Liu, Y.-Z.; Shen, Z.; Qiao, Z.; Ma, X. Regioselective synthesis of pyrazolo[1,5-a] pyridine via TEMPO-mediated [3 + 2] annulation-aromatization of N-aminopyridines and α,β-unsaturated compounds. Org. Lett. 2022, 24, 1454–1459; (d) Ling, L.; Chen, J.; Song, J.; Zhang, Y.; Li, X.; Song, L.; Shi, F.; Li, Y.; Wu, C. From N-Benzoylpyridinium Imides to Pyrazolo[1,5-a] pyridines: A Mechanistic Discussion on a Stoichiometric Cu Protocol. Org. Biomol. Chem. 2013, 11, 3894–3902; (e) Ravi, C.; Samanta, S.; Mohan, D. C.; Reddy, N. N. K.; Adimurthy, S. Synthesis of Functionalized Pyrazolo[1,5-a]pyridines: [3 + 2] Cycloaddition of N-Aminopyridines and α,β-Unsaturated Carbonyl Compounds/Alkenes at Room Temperature. Synthesis 2017, 49, 2513–2522.
- 7(a) Dai, W.; Li, C.; Liu, Y.; Han, X.; Li, X.; Chen, K.; Liu, H. Palladium- Catalyzed [4 + 3] Dearomatizing Cycloaddition Reaction of N-iminoquinolinium Ylides. Org. Chem. Front. 2020, 7, 2612–2617; (b) Mousseau, J. J.; Bull, J. A.; Ladd, C. L.; Fortier, A.; Sustac Roman, D.; Charette, A. B. Synthesis of 2- and 2,3-Substituted Pyrazolo[1,5-a]Pyridines: Scope and Mechanistic Considerations of a Domino Direct Alkynylation and Cyclization of N-Iminopyridinium Ylides Using Alkenyl Bromides, Alkenyl Iodides, and Alkynes. J. Org. Chem. 2011, 76, 8243–8261; (c) Ding, S.; Yan, Y.; Jiao, N. Copper-Catalyzed Direct Oxidative Annulation of N-Iminopyridinium Ylides with Terminal Alkynes Using O2 as Oxidant. Chem. Commun. 2013, 49, 4250–4252; (d) Ravi, C.; Mohan, D. C.; Reddy, N. N. K.; Adimurthy, S. Substrate Selective Synthesis of Pyrazolo[1,5-a]pyridines through [3 + 2] Cycloaddition of N-Aminopyridines and β-Nitro Styrenes. RSC Adv. 2015, 5, 42961–42964.
- 8(a) Li, W.; Zhang, M.; Yan, J.; Ni, L.; Cao, H.; Liu, X. Transition metal- and oxidant-free [3 + 2] cyclization of azomethine imines utilizing vinylene carbonate as dual synthons. Org. Chem. Front. 2022, 9, 2529–2533;
(b) Shi, X.; Lin, Y.; Wei, J.; Zhao, L.; Guo, P.; Cao, H.; Liu, X. One-step synthesis of cyanated pyrazolo[1,5-a]pyridines utilizing N-aminopyridines as a 1,3-dipole and a nitrogen source. Org. Chem. Front. 2023, 10, 2892–2897;
(c) Shi, X.; Wang, Q.; Tang, Z.; Huang, H.; Cao, T.; Cao, H.; Liu, X. Divergent Synthesis of F- and CF3-Containing N-Fused Heterocycles Enabled by Fragmentation Cycloaddition of β-CF3–1,3-Enynes with N-Aminopyridiniums Ylides. Org. Lett. 2024, 26, 1255−1260;
(d) Zhu, B.; Li, W.; Chen, H.; Wu, M.; Hu, J.; Cao, H.; Liu, X. Mechanochemical Synthesis of 1,2,4-Triazoles via a [3 + 2] Cycloaddition of Azinium-N-Imines and Nitriles. Adv. Synth. Catal. 2022, 364, 2911–2915;
(e) Ma, S.; Chen, M.; Yang, Z.; Liu, X.; Cao, H. DBU-Promoted [3+2] Cyclization/Retro-Mannich Cascade Reaction of N-Aminoisoquinolinium and N-Aminoquinolinium Derivatives with para-Quinone Methides. Adv. Synth. Catal. 2024, 366, 2003–2007;
(f) Liu, X.; Li, W.; Jiang, W.; Lu, H.; Liu, J.; Lin, Y.; Cao, H. Cu(II)-Catalyzed C–H Amidation/Cyclization of Azomethine Imines with Dioxazolones via Acyl Nitrenes: A Direct Access to Diverse 1,2,4-Triazole Derivatives. Org. Lett. 2022, 22, 613–618;
10.1021/acs.orglett.1c04044 Google Scholar(g) Zhao, L.; Li, W.; Liu, J.; Ni, L.; Liu, Z.; Shen, H.; Cao, H.; Liu, X. Transition metal-free annulative vinylene transfer via the 1,3-dipolar reaction of N-ylides: access to benzo-fused indolizines. Org. Biomol. Chem. 2022, 20, 9604–9608.
- 9(a) Lober, S.; Hubner, H.; Gmeiner, P. Fused Azaindole Derivatives: Molecular Design, Synthesis and In Vitro Pharmacology Leading to the Preferential Dopamine D3 Receptor Agonist FAUC 725. Bioorg. Med. Chem. Lett. 2002, 12, 2377–2380; (b) Bettinetti, L.; Schlotter, K.; Hubner, H.; Gmeiner, P. Interactive SAR Studies: Rational Discovery of Super-Potent and Highly Selective Dopamine D3 Receptor Antagonists and Partial Agonists. J. Med. Chem. 2002, 45, 4594–4597; (c) Johns, B. A.; Gudmundsson, K. S.; Turner, E. M.; Allen, S. H.; Samano, V. A.; Ray, J. A.; Freeman, G. A.; Sexton, C. J.; Selleseth, D. W.; Creech, K. L.; Moniri, K. R. Pyrazolopyridine antiherpetics: SAR of C2’ and C7 amine substituents. Bioorg. Med. Chem. 2005, 13, 2397–2411; (d) Takahashi, Y.; Hibi, S.; Hoshino, Y.; Kikuchi, K.; Shin, K.; Murata-Tai, K.; Fujisawa, M.; Ino, M.; Shibata, H.; Yonaga, M. Synthesis and Structure-Activity Relationships of Pyrazolo[1,5-a]pyridine Derivatives: Potent and Orally Active Antagonists of Corticotropin- Releasing Factor 1 Receptor. J. Med. Chem. 2012, 55, 5255–5269; (e) Kendall, J. D.; Marshall, A. J.; Giddens, A. C.; Tsang, K. Y.; Boyd, M.; Frederick, R.; Lill, C. L.; Lee, W.-J.; Kolekar, S.; Chao, M.; Malik, A.; Yu, S.; Chaussade, C.; Buchanan, C. M.; Rewcastle, G. W.; Baguley, B. C.; Flanagan, J. U.; Denny, W. A.; Shepherd, P. R. Novel pyrazolo[1,5-a]pyridines as PI3K inhibitors: variation of the central linker group. Med. Chem. Commun. 2014, 5, 41–46.
- 10(a) Xiong, Y.; Ullman, B.; Choi, J.-S. K.; Cherrier, M.; Strah-Pleynet, S.; Decaire, M.; Feichtinger, K.; Frazer, J. M.; Yoon, W. H.; Whelan, K.; Sanabria, E. K.; Grottick, A. J.; Al-Shamma, H.; Semple, G. Identification of fused bicyclic heterocycles as potent and selective 5-HT2A receptor antagonists for the treatment of insomnia. Bioorg. Med. Chem. Lett. 2012, 22, 1870–1873; (b) Tai, V. W. F.; Garrido, D.; Price, J.; Maynard, A.; Pouliot, J. J.; Xiong, Z.; Seal, J. W.; Creech, K.; Kryn, L. L. H.; Baughman, T. M.; Peat, A. J. Design and synthesis of spirocyclic compounds as HCV replication inhibitors by targeting viral NS4B protein. Bioorg. Med. Chem. Lett. 2014, 24, 2288–2294; (c) Zhou, H.-J.; Wang, J.; Yao, B.; Wong, S.; Djakovic, S.; Kumar, B.; Rice, J.; Valle, E.; Soriano, F.; Menon, M.-K.; Madriaga, A.; Kiss von Soly, S.; Kumar, A.; Parlati, F.; Yakes, F. M.; Shawver, L.; Le Moigne, R.; Anderson, D. J.; Rolfe, M.; D. Wustrow, M. Discovery of a first-in- class, potent, selective, and orally bioavailable inhibitor of the p97 AAA ATPase (CB-5083). J. Med. Chem. 2015, 58, 9480–9497; (d) Nishigaya, Y.; Umei, K.; Saito, Y.; Watanabe, H.; Kondo, T.; Kondo, A.; Kawamura, N.; Tatani, K.; Kohno, Y.; Tanaka, N.; Seto, S. Discovery of novel pyrazolo[1,5-a]pyridine-based EP1 receptor antagonists by scaffold hopping: Design, synthesis, and structure-activity relationships. Bioorg. Med. Chem. Lett. 2017, 27, 4044–4050; (e) Mikami, S.; Sasaki, S.; Asano, Y.; Ujikawa, O.; Fukumoto, S.; Nakashima, K.; Oki, H.; Kamiguchi, N.; Imada, H.; Iwashita, H.; Taniguchi, T. Discovery of an orally bioavailable, brain-penetrating, in vivo active phosphodiesterase 2A inhibitor lead series for the treatment of cognitive disorders. J. Med. Chem. 2017, 60, 7658–7676.
- 11(a) Yan, J.; Zhong, S.; Chen, X.; Luo, Y.; Cao, H.; Liu, X.; Zhao, L. Controlled and Site-Selective C−H/N−H Alkenylation, Dialkenylation, and Dehydrogenative β-Alkenylation of Various N-Heterocycles. J. Org. Chem. 2024, 89, 4840−4850; (b) Passia, M.; Schöbel, J.; Lentelink, N. J.; Truong, K.; Rissanen, K.; Bolm, C. Synthesis of trifluoromethyl-substituted 1,2,6-thiadiazine 1-oxides from sulfonimidamides under mechanochemical conditions. Org. Biomol. Chem. 2021, 19, 9470–9475.
- 12(a) Chen, X. Y.; Zhang, X.; Wan, J.-P. Recent advances in transition metal-free annulation toward heterocycle diversity based on the C–N bond cleavage of enaminone platform. Org. Biomol. Chem. 2022, 20, 2356–2369; (b) Zhang, M.; Chen, L.; Sun, H.; Liu, Z.; Yan, S.-J.; Yu, F. Rh(III)-Catalyzed [3 + 2] Annulation/Pinacol Rearrangement Reaction of Enaminones with Iodonium Ylides: Direct Synthesis of 2-Spirocyclo-pyrrol-3-ones. Org. Lett. 2023, 25, 7214–7219; (c) Lei, S.-G.; Zhou, Y.; Wang, L.-S.; Yu, Z.-C.; Chen, T.; Wu, Y.-D.; Gao, M.; Wu, A.-X. I2-DMSO mediated dual α,β-C(sp2)-H functionalization/bicyclization of o-hydroxyphenyl enaminones to construct C2,C3-disubstituted chromone derivatives: chromeno[2,3-b]pyrrol-4(1H)-ones. Org. Chem. Front. 2023, 10, 4843–4847; (d) Yang, C.; Zhang, X.; Fan, X. Synthesis of Homophthalimide Spironaphthalenones Through [5 + 1] Spiroannulation of Aryl/alkenyl Enaminones with Diazo Homophthalimides. Org. Chem. Front. 2023, 10, 4282–4288.
- 13(a) Huang, L.; Yu, F. Recent Advances in Organic Synthesis Based on N,N-Dimethyl Enaminones. Synthesis-Stuttgart 2021, 53, 587–610; (b) Zhang, M.; Chen, L.; Sun, H.; Liu, Z.; Huang, J.; Yu, F. Synthesis of Tetrahydro-indolones through Rh(III)-Catalyzed [3 + 2] Annulation of Enaminones with Iodonium Ylides. Org. Lett. 2023, 25, 7298–7303; (c) Zhang, M.; Chen, L.; Liu, Z.; Huang, J.; Yu, F. Unprecedented chemoselective Ru(III)-catalyzed [3 + 2] annulation of enaminones with iodonium ylides for the synthesis of functionalized 3a,7a-dihydroxy hexahydro-4H-indol-4-ones. Org. Chem. Front. 2023, 10, 5660–5666; (d) Song, S.; Peng, M.; Zhang, Z.; Hu, H.; Wei, Y.; Yan, S.; Wang, Y.; Yu, F. Divergent Synthesis of 2-Chromonyl-3-hydrazono-chromones and 2-Alkoxy-3-hydrazono-chromones through Switchable Annulation Reactions of o-Hydroxyphenylenaminones with Aryldiazonium Salts. Org. Lett. 2024, 26, 4980–4985; (e) Song, S.; Zhang, Z.; Peng, M.; Xia, X.; Dong, S.; Wang, Y.; Yu, F. Selective synthesis of pyridazine-fused chromones and 3-pyridazinyl chromones through intermolecular chromone annulation of o-hydroxyphenylenaminones with aryldiazonium salts. Org. Chem. Front. 2024, 11, 3906–3912; (f) Han, Y.; Zhou, L.; Wang, C.; Feng, S.; Ma, R.; Wan, J. Recent advances in visible light-mediated chemical transformations of enaminones. Chin. Chem. Lett. 2024, 35, 108977.
- 14(a) Wu, W.; Jiang, H. Haloalkynes: A Powerful and Versatile Building Block in Organic Synthesis. Acc. Chem. Res. 2014, 47, 2483–2504; (b) Li, M.; Fang, S.; Zheng, J.; Jiang, H.; Wu, W. Direct Assembly of Polysubstituted Propiolamidinates via Palladium-Catalyzed Multicomponent Reaction of Isocyanides. Org. Lett. 2019, 21, 8439–8443; (c) Fang, S.; Jiang, H.; Wu, W. Palladium-Catalyzed Tandem Cyclization Strategy for the Assembly of 3-Halo-1,2,5-triarylpyrroles from N-Alkylanilines and Haloalkynes. Chin. J. Chem. 2023, 41, 181–187; (d) Peng, X.; Hong, H.; Wang, L.; Chen, L.; Peng, J.; Li, Y.; Jiang, H. Palladium-catalyzed multi-component Heck alkynylcarbonylation of unactivated alkenes for synthesis of β,γ-alkynoates. Org. Chem. Front. 2024, 11, 3451–3458; (e) Fang, S.; Jiang, H.; Wu, W.; Palladium-Catalyzed Tandem Cyclization Strategy for the Assembly of 3-Halo-1,2,5- triarylpyrroles from N-Alkylanilines and Haloalkynes. Chin. J. Chem. 2023, 41, 181–187.




