Hyperkouytones A—O, New Polyprenylated Acylphloroglucinols from Hypericum kouytchense with Multidrug Resistance Reversal Activity
Hua-Yong Lou
State Key Laboratory of Functions and Applications of Medicinal Plants, Guizhou Medical University, Guiyang, Guizhou, 550014 China
Natural Products Research Center, Guiyang, Guizhou, 550014 China
School of Pharmaceutical Sciences, Guizhou Medical University, Guiyang, Guizhou, 561113 China
Those authors contribute equally.
Search for more papers by this authorMei-Jun Chen
State Key Laboratory of Functions and Applications of Medicinal Plants, Guizhou Medical University, Guiyang, Guizhou, 550014 China
Natural Products Research Center, Guiyang, Guizhou, 550014 China
School of Pharmaceutical Sciences, Guizhou Medical University, Guiyang, Guizhou, 561113 China
Those authors contribute equally.
Search for more papers by this authorPing Yi
State Key Laboratory of Functions and Applications of Medicinal Plants, Guizhou Medical University, Guiyang, Guizhou, 550014 China
Natural Products Research Center, Guiyang, Guizhou, 550014 China
School of Pharmaceutical Sciences, Guizhou Medical University, Guiyang, Guizhou, 561113 China
Search for more papers by this authorJun Jin
State Key Laboratory of Functions and Applications of Medicinal Plants, Guizhou Medical University, Guiyang, Guizhou, 550014 China
Natural Products Research Center, Guiyang, Guizhou, 550014 China
School of Pharmaceutical Sciences, Guizhou Medical University, Guiyang, Guizhou, 561113 China
Search for more papers by this authorYan-Rong Zeng
State Key Laboratory of Functions and Applications of Medicinal Plants, Guizhou Medical University, Guiyang, Guizhou, 550014 China
Natural Products Research Center, Guiyang, Guizhou, 550014 China
School of Chinese Ethnic Medicine, Guizhou Minzu University, Guiyang, Guizhou, 550025 China
Search for more papers by this authorWei Gu
State Key Laboratory of Functions and Applications of Medicinal Plants, Guizhou Medical University, Guiyang, Guizhou, 550014 China
Natural Products Research Center, Guiyang, Guizhou, 550014 China
School of Pharmaceutical Sciences, Guizhou Medical University, Guiyang, Guizhou, 561113 China
Search for more papers by this authorZhan-Xing Hu
State Key Laboratory of Functions and Applications of Medicinal Plants, Guizhou Medical University, Guiyang, Guizhou, 550014 China
Natural Products Research Center, Guiyang, Guizhou, 550014 China
School of Pharmaceutical Sciences, Guizhou Medical University, Guiyang, Guizhou, 561113 China
Search for more papers by this authorCorresponding Author
Jue Yang
State Key Laboratory of Functions and Applications of Medicinal Plants, Guizhou Medical University, Guiyang, Guizhou, 550014 China
Natural Products Research Center, Guiyang, Guizhou, 550014 China
School of Pharmaceutical Sciences, Guizhou Medical University, Guiyang, Guizhou, 561113 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Xiao-Jiang Hao
State Key Laboratory of Functions and Applications of Medicinal Plants, Guizhou Medical University, Guiyang, Guizhou, 550014 China
Natural Products Research Center, Guiyang, Guizhou, 550014 China
School of Pharmaceutical Sciences, Guizhou Medical University, Guiyang, Guizhou, 561113 China
State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Kunming, Yunnan, 650201 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Chun-Mao Yuan
State Key Laboratory of Functions and Applications of Medicinal Plants, Guizhou Medical University, Guiyang, Guizhou, 550014 China
Natural Products Research Center, Guiyang, Guizhou, 550014 China
School of Pharmaceutical Sciences, Guizhou Medical University, Guiyang, Guizhou, 561113 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorHua-Yong Lou
State Key Laboratory of Functions and Applications of Medicinal Plants, Guizhou Medical University, Guiyang, Guizhou, 550014 China
Natural Products Research Center, Guiyang, Guizhou, 550014 China
School of Pharmaceutical Sciences, Guizhou Medical University, Guiyang, Guizhou, 561113 China
Those authors contribute equally.
Search for more papers by this authorMei-Jun Chen
State Key Laboratory of Functions and Applications of Medicinal Plants, Guizhou Medical University, Guiyang, Guizhou, 550014 China
Natural Products Research Center, Guiyang, Guizhou, 550014 China
School of Pharmaceutical Sciences, Guizhou Medical University, Guiyang, Guizhou, 561113 China
Those authors contribute equally.
Search for more papers by this authorPing Yi
State Key Laboratory of Functions and Applications of Medicinal Plants, Guizhou Medical University, Guiyang, Guizhou, 550014 China
Natural Products Research Center, Guiyang, Guizhou, 550014 China
School of Pharmaceutical Sciences, Guizhou Medical University, Guiyang, Guizhou, 561113 China
Search for more papers by this authorJun Jin
State Key Laboratory of Functions and Applications of Medicinal Plants, Guizhou Medical University, Guiyang, Guizhou, 550014 China
Natural Products Research Center, Guiyang, Guizhou, 550014 China
School of Pharmaceutical Sciences, Guizhou Medical University, Guiyang, Guizhou, 561113 China
Search for more papers by this authorYan-Rong Zeng
State Key Laboratory of Functions and Applications of Medicinal Plants, Guizhou Medical University, Guiyang, Guizhou, 550014 China
Natural Products Research Center, Guiyang, Guizhou, 550014 China
School of Chinese Ethnic Medicine, Guizhou Minzu University, Guiyang, Guizhou, 550025 China
Search for more papers by this authorWei Gu
State Key Laboratory of Functions and Applications of Medicinal Plants, Guizhou Medical University, Guiyang, Guizhou, 550014 China
Natural Products Research Center, Guiyang, Guizhou, 550014 China
School of Pharmaceutical Sciences, Guizhou Medical University, Guiyang, Guizhou, 561113 China
Search for more papers by this authorZhan-Xing Hu
State Key Laboratory of Functions and Applications of Medicinal Plants, Guizhou Medical University, Guiyang, Guizhou, 550014 China
Natural Products Research Center, Guiyang, Guizhou, 550014 China
School of Pharmaceutical Sciences, Guizhou Medical University, Guiyang, Guizhou, 561113 China
Search for more papers by this authorCorresponding Author
Jue Yang
State Key Laboratory of Functions and Applications of Medicinal Plants, Guizhou Medical University, Guiyang, Guizhou, 550014 China
Natural Products Research Center, Guiyang, Guizhou, 550014 China
School of Pharmaceutical Sciences, Guizhou Medical University, Guiyang, Guizhou, 561113 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Xiao-Jiang Hao
State Key Laboratory of Functions and Applications of Medicinal Plants, Guizhou Medical University, Guiyang, Guizhou, 550014 China
Natural Products Research Center, Guiyang, Guizhou, 550014 China
School of Pharmaceutical Sciences, Guizhou Medical University, Guiyang, Guizhou, 561113 China
State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Kunming, Yunnan, 650201 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Chun-Mao Yuan
State Key Laboratory of Functions and Applications of Medicinal Plants, Guizhou Medical University, Guiyang, Guizhou, 550014 China
Natural Products Research Center, Guiyang, Guizhou, 550014 China
School of Pharmaceutical Sciences, Guizhou Medical University, Guiyang, Guizhou, 561113 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorComprehensive Summary
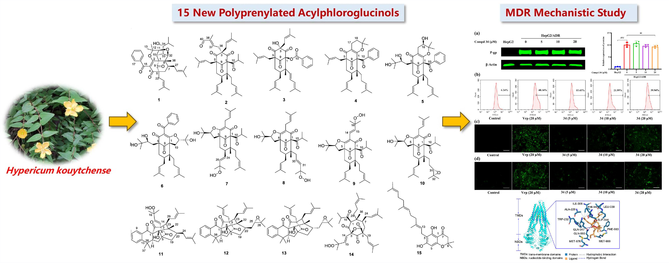
Given the lack of systematic polyprenylated acylphloroglucinols (PPAPs) research on traditional Chinese medicine of Hypericum kouytchense, this plant was applied for the phytochemical study, which led to fifteen new PPAPs (1—15, PPAPs), along with 36 known PPAP derivatives. Their structures and absolute configurations were established by comprehensive spectral analysis and theoretical ECD and NMR calculations. Structurally, compound 1 possesses a rare fused 6/6/6/5/5 pentacyclic ring system. Eleven compounds exhibited good multidrug resistance reversal activity (RF ranging from 5 to 53) in HepG2/ADR cells. Importantly, compound 34, the most potential MDR modulator, showed better reversal effect (RF: 53) than positive control, verapamil. The primary mechanistic study of compound 34, implied that this compound could prohibit the function of P-gp transport rather than its expression. The possible recognition mechanism between compound 34 and P-gp was predicted by molecular docking.
Supporting Information
| Filename | Description |
|---|---|
| cjoc_202400739_sm_suppl.pdfPDF document, 7.9 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Han, B.; Zheng, R.; Zeng, H.; Wang, S.; Sun, K.; Chen, R.; Li, L.; Wei, W.; He, J. Cancer Incidence and Mortality in China, 2022. J. Natl. Cancer Cent. 2024, 4, 47–53.
- 2 Wang, W.; Dong, X.; Liu, Y.; Ni, B.; Sai, N.; You, L.; Sun, M.; Yao, Y.; Qu, C.; Yin, X.; Ni, J. Itraconazole Exerts Anti-liver Cancer Potential through the Wnt, PI3K/AKT/mTOR, and ROS Pathways. Biomed. Pharmacother. 2020, 131, 110661.
- 3 Singh, A. K.; Singh, S. V.; Kumar, R.; Kumar, S.; Senapati, S.; Pandey, A. K. Current Therapeutic Modalities and Chemopreventive Role of Natural Products in Liver Cancer: Progress and Promise. World J. Hepatol. 2023, 15, 1.
- 4 Galicia-Moreno, M.; Silva-Gomez, J. A.; Lucano-Landeros, S.; Santos, A.; Monroy-Ramirez, H. C.; Armendariz-Borunda, J. Liver Cancer: Therapeutic Challenges and the Importance of Experimental Models. Can. J. Gastroenterol. Hepatol. 2021, 2021, 8837811.
- 5 Shah, D.; Ajazuddin, B. S. Role of Natural p-gp Inhibitor in the Effective Delivery for Chemotherapeutic Agents. J. Cancer Res. Clin. Oncol. 2023, 149, 367–391.
- 6 Elmeliegy, M.; Vourvahis, M.; Guo, C.; Wang, D. D. Effect of P-Glycoprotein (p-gp) Inducers on Exposure of p-Gp Substrates: Review of Clinical Drug-drug Interaction Studies. Clin. Pharmacokinet. 2020, 59, 699–714.
- 7 Hasan, A.; Tang, D.; Nijat, D.; Yang, H.; Aisa, H. A. Diterpenoids from Euphorbia Glomerulans with Potential Reversal Activities against p-Glycoprotein-mediated Multidrug Resistance. Bioorg. Chem. 2021, 117, 105442.
- 8 Zeng, Y. R.; Li, Y. N.; Yang, J.; Yi, P.; Huang, L.; Huang, L. J.; Gu, W.; Hu, Z. X.; Li, Y. M.; Yuan, C. M.; Hao, X. J. Hypermonones A—I, New Polyprenylated Acylphloroglucinols from Hypericum Monogynum with Multidrug Resistance Reversal Activity. Chin. J. Chem. 2021, 39, 2422–2432.
- 9 Velingkar, V. S.; Dandekar, V. D. Design, Synthesis and Evaluation of Substituted N-(3-arylpropyl)-9,10-dihydro-9-oxoacridine-4-carboxamides as Potent MDR Reversal Agents in Cancer. Chin. J. Chem. 2011, 29, 504–510.
- 10 Teixeira, R. G.; Salaroglio, I. C.; Oliveira, N. F. B.; Sequeira, J. G. N.; Fontrodona, X.; Romero, I.; Machuqueiro, M.; Tomaz, A. I.; Garcia, M. H.; Riganti, C.; Valente, A. Fighting Multidrug Resistance with Ruthenium–cyclopentadienyl Compounds: Unveiling the Mechanism of p-Gp Inhibition. J. Med. Chem. 2023, 66, 14080–14094.
- 11 Qiu, Q.; Zou, F.; Li, H.; Shi, W.; Zhou, D.; Zhang, P.; Li, T.; Yin, Z.; Cai, Z.; Jiang, Y.; Huang, W.; Qian, H. Structure-based Discovery of Pyrimidine Aminobenzene Derivatives as Potent Oral Reversal Agents against p-Gp- and BCRP-mediated Multidrug Resistance. J. Med. Chem. 2021, 64, 6179–6197.
- 12 Newman, D. J.; Cragg, G. M. Natural Products as Sources of New Drugs Over the 30 Years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335.
- 13 Wang, S.; Dong, G.; Sheng, C. Structural Simplification of Natural Products. Chem. Rev. 2019, 119, 4180–4220.
- 14 Yang, X. W.; Li, M. M.; Liu, X.; Ferreira, D.; Ding, Y.; Zhang, J. J.; Liao, Y.; Qin, H. B.; Xu, G. Polycyclic Polyprenylated Acylphloroglucinol Congeners Possessing Diverse Structures from Hypericum henryi. J. Nat. Prod. 2015, 78, 885–895.
- 15 Ye, Y. S.; Liu, R.; Jiang, N. N.; Li, S. Y.; Nian, Y.; Xu, G. Caged Polycyclic Polyprenylated Acylphloroglucinols as Cav3.2 Low Voltage-gated Ca2+ Channel Inhibitors from Hypericum curvisepalum. Chem. Commun. 2022, 58, 13135–13138.
- 16 Lu, W. J.; Xu, W. J.; Zhang, M. H.; Zhang, Y. Q.; Li, Y. R.; Zhang, H.; Luo, J.; Kong, L. Y. Diverse Polycyclic Polyprenylated Acylphloroglucinol Congeners with Anti-nonalcoholic Steatohepatitis Activity from Hypericum forrestii. J. Nat. Prod. 2021, 84, 1135–1148.
- 17 Jiang, N. N.; Ye, Y. S.; Liu, X.; Wang, Y. L.; Xu, G. Rearranged Homoadamantane-type Polycyclic Polyprenylated Acylphloroglucinols from Hypericum pseudohenryi. Org. Lett. 2023, 25, 8965–8969.
- 18 Yang, X. W.; Grossman, R. B.; Xu, G. Research Progress of Polycyclic Polyprenylated Acylphloroglucinols. Chem. Rev. 2018, 118, 3508–3558.
- 19 Ting, C. P.; Maimone, T. J. Total Synthesis of Hyperforin. J. Am. Chem. Soc. 2015, 137, 10516–10519.
- 20 Li, S.; Chen, Q.; Xie, X.; Yang, J.; Zhang, J. Pd-catalyzed Enantioselective Dearomative Allylic Annulation to Access PPAPs Analogues. Org. Lett. 2021, 23, 7824–7828.
- 21 Huang, M.; Chen, L. Z.; Hu, J. B.; He, S. Z. Species and Distribution of Medicinal Plants of the Genus Hypericum in Guizhou. China J. Chin. Mater. Med. 2000, 25, 458–461.
- 22 Erdelmeier, C. A. J. Hyperforin, Possibly the Major Non-nitrogenous Secondary Metabolite of Hypericum perforatum L. Pharmacopsychiatry 1998, 31, 2–6.
- 23 Verotta, L.; Appendino, G.; Jakupovic, J.; Bombardelli, E. Hyperforin Analogues from St. John's Wort (Hypericum perforatum). J. Nat. Prod. 2000, 63, 412.
- 24 Liao, Y.; Yang, S. Y.; Li, X. N.; Yang, X. W.; Xu, G. Polyprenylated Acylphloroglucinols from the Fruits of Hypericum Henryi. Sci. China Chem. 2016, 59, 1216–1223.
- 25 Zhou, Z. B.; Li, Z. R.; Wang, X. B.; Luo, J. G.; Kong, L. Y. Polycyclic Polyprenylated Derivatives from Hypericum Uralum: Neuroprotective Effects and Antidepressant-like Activity of Uralodin A. J. Nat. Prod. 2016, 79, 1231–1240.
- 26 Liu, X.; Yang, X. W.; Chen, C. Q.; Wu, C. Y.; Zhang, J. J.; Ma, J. Z.; Wang, H.; Yang, L. X.; Xu, G. Bioactive Polyprenylated Acylphloroglucinol Derivatives from Hypericum cohaerens. J. Nat. Prod. 2013, 76, 1612–1618.
- 27 Lee, J. Y.; Duke, R. K.; Tran, V. H.; Hook, J. M.; Duke, C. C. Hyperforin and Its Analogues Inhibit CYP3A4 Enzyme Activity. Phytochemistry 2006, 67, 2550–2560.
- 28 Guo, N.; Chen, X. Q.; Zhao, Q. S. A New Polyisoprenylated Benzoylphloroglucinol Derivative from Hypericum Henryi. Acta Bontanica Yunnanica 2008, 30, 515–518.
- 29 Yang, X. W.; Ding, Y.; Zhang, J. J.; Liu, X.; Yang, L. X.; Li, X. N.; Ferreira, D.; Walker, L. A.; Xu. G. New Acylphloroglucinol Derivatives with Diverse Architectures from Hypericum henryi. Org. Lett. 2014, 16, 2434–2437.
- 30 Li, X.; Li, Y.; Luo, J.; Zhou, Z.; Xue, G.; Kong, L. Y. New Phloroglucinol Derivatives from the Whole Plant of Hypericum uralum. Fitoterapia 2017, 123, 59–64.
- 31 Zhang, J. J.; Yang, X. W.; Liu, X.; Ma, J. Z.; Liao, Y.; Xu, G. 1,9-Seco-bicyclic Polyprenylated Acylphloroglucinols from Hypericum uralum. J. Nat. Prod. 2015, 78, 3075–3079.
- 32 Verotta, L.; Lovaglio, E.; Sterner, O.; Appendino, G.; Bombardelli, E. Oxidative Fragmentation of the Bridged β-Triketone Core of Hyperforin. Eur. J. Org. Chem. 2004, 2004, 1193–1197.
- 33 Zhang, J. J.; Yang, J.; Liao, Y.; Yang, X. W.; Ma, J. Z.; Xiao, Q. L.; Yang, L. X.; Xu, G. Hyperuralones A and B, New Acylphloroglucinol Derivatives with Intricately Caged Cores from Hypericum uralum. Org. Lett. 2014, 16, 4912–4915.
- 34 Zhou, Z. B.; Zhang, Y. M.; Pan, K.; Luo, J. G.; Kong, L. Y. Cytotoxic Polycyclic Polyprenylated Acylphloroglucinols from Hypericum attenuatum. Fitoterapia 2014, 95, 1–7.
- 35 Hashida, C.; Tanaka, N.; Kawazoe, K.; Murakami, K.; Sun, H. D.; Takaishi, Y.; Kashiwada, Y. Hypelodins A and B, Polyprenylated Benzophenones from Hypericum elodeoides. J. Nat. Med. 2014, 68, 737–742.
- 36 Hu, L. H.; Sim, K. Y. Sampsoniones C–H, a Unique Family of Polyprenylated Benzophenone Derivatives with the Novel Tetracyclo [7.3. 1.13,11.03,7] Tetradecane-2,12,14-trione Skeleton, from Hypericum sampsonii (Guttiferae). Tetrahedron Lett. 1999, 40, 759–762.
- 37 Ishida, Y.; Shirota, O.; Sekita, S.; Someya, K.; Tokita, F.; Nakane, T.; Kuroyanagi, M. Polyprenylated Benzoylphloroglucinol-type Derivatives Including Novel Cage Compounds from Hypericum erectum. Chem. Pharma. Bull. 2010, 58, 336–343.
- 38 Xu, W. J.; Tang, P. F.; Lu, W. J.; Zhang, Y. Q.; Wang, X. B.; Zhang, H.; Luo, J.; Kong, L. Y. Hyperberins A and B, Type B Polycyclic Polyprenylated Acylphloroglucinols with Bicyclo[5.3.1]hendecane Core from Hypericum beanie. Org. Lett. 2019, 21, 8558–8562.
- 39 Zhang, X. Q.; Ye, W. C.; Zhang, Z. H.; Zhao, S. X. Studies on Chemical Constituent of Olanum Lyratum. China J. Chin. Mater. Med. 2005, 30, 791–792.
- 40 Shan, M. D.; Hu, L. H.; Chen, Z. L. Three New Hyperforin Analogues from Hypericum perforatum. J. Nat. Prod. 2001, 64, 127–130.
- 41 Yang, X. W.; Yang, J.; Liao, Y.; Ye, Y.; Li, Y. P.; Yang, S. Y.; Xia, F.; Xu, G. Hypercohin K, a Polycyclic Polyprenylated Acylphloroglucinol with an Unusual Spiro-fused Cyclopropane Ring from Hypericum cohaerens. Tetrahedron Lett. 2015, 56, 5537–5540.
- 42 Zhou, K.; Wunsch, C.; Dai, J.; Li, S. M. Gem-diprenylation of Acylphloroglucinols by a Fungal Prenyltransferase of the Dimethylallyltryptophan Synthase Superfamily. Org. Lett. 2017, 19, 388–391.
- 43 Ye, Y.; Yang, X. W.; Xu, G. Unusual Adamantane Type Polyprenylated Cylphloroglucinols with an Oxirane Unit and Their Structural Transformation from Hypericum hookerianum. Tetrahedron 2016, 72, 3057–3062.
- 44 Liao, Y., Yang, S. Y.; Li, X. N.; Yang, X. W.; Xu, G. Polyprenylated Acylphloroglucinols from the Fruits of Hypericum henryi. Sci. China Chem. 2016, 59, 1216–1223.
- 45 Yang, C. S.; Jiang, H. L.; Li, H. Y.; Dong, X. A Unique Acyclic Sesquiterpene-flavanone Adduct and a New Cyclic Diarylheptanoid from Alpinia katsumadai. Phytochem. Lett. 2019, 30, 190–193.
- 46 Zeng, Y. R.; Li, Y. N.; Zhang, Z. Z.; Hu, Z. X.; Gu, W.; Huang, L. J.; Li, Y. M.; Yuan, C. M.; Hao, X. J. Hypermoins A–D: Rearranged Nor-polyprenylated Acylphloroglucinols from the Flowers of Hypericum monogynum. J. Org. Chem. 2021, 86, 7021–7027.
- 47 Zeng, Y.; Yang, J.; Li, Y.; Gu, W.; Huang, L.; Yi, P.; Yuan, C.; Hao, X. Hypermogins A–D, Four Highly Modified Polycyclic Polyprenylated Acylphloroglucinols from Hypericum monogynum. Tetrahedron Lett. 2021, 64, 152733.
- 48 Zhang, Z. Z.; Zeng, Y. R.; Li, Y. N.; Hu, Z. X.; Huang, L. J.; Gu, W.; Hao, X. J.; Yuan, C. M. Two New Seco-polycyclic Polyprenylated Acylphloroglucinol from Hypericum sampsonii. Org. Biomol. Chem. 2021, 19, 216–219.
- 49 Shaker, S.; Sang, J.; Yan, X. L.; Fan, R. Z.; Tang, G. H.; Xu, Y. K.; Yin, S. Diterpenoids from Euphorbia Royleana Reverse p-Glycoprotein-mediated Multidrug Resistance in Cancer Cells. Phytochemistry 2020, 176, 112395.
- 50 Zhu, J.; Wang, R.; Lou, L.; Li, W.; Tang, G.; Bu, X.; Yin, S. Jatrophane Diterpenoids as Modulators of p-Glycoprotein-dependent Multidrug Resistance (MDR): Advances of Structure–Activity Relationships and Discovery of Promising MDR Reversal Agents. J. Med. Chem. 2016, 59, 6353–6369.
- 51 Yang, H.; Mamatjan, A.; Tang, D.; Aisa, H. A. Jatrophane Diterpenoids as Multidrug Resistance Modulators from Euphorbia sororia. Bioorg. Chem. 2021, 112, 104989.
- 52 Xiao, Y. Z.; Muhammad, I.; Ma, X. P.; Yu, H. J.; Yan, S. K.; Xiao, X.; Jin, H. Z. Camganoids A and B, Two New Sesquiterpenes with Different Carbon Skeletons Isolated from Fruits of Cinnamomum migao. Chin. Herb. Med. 2022, 14, 638–642.




