Enhanced Efficiency of Amide-Substituted Quinuclidine-Boranes as Hydridic Hydrogen Atom Transfer Catalysts for Photoinduced Hydroalkylation of Unactivated Olefins†
Xiao Yang
Inner Mongolia Key Laboratory of Synthesis and Application of Organic Functional Molecules, College of Chemistry and Chemical Engineering, Inner Mongolia University, Hohhot, Inner Mongolia, 010021 China
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, Shanghai, 200240 China
Search for more papers by this authorMengdi Ma
Inner Mongolia Key Laboratory of Synthesis and Application of Organic Functional Molecules, College of Chemistry and Chemical Engineering, Inner Mongolia University, Hohhot, Inner Mongolia, 010021 China
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, Shanghai, 200240 China
Search for more papers by this authorMeichen Xu
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, Shanghai, 200240 China
Search for more papers by this authorYubing Pang
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, Shanghai, 200240 China
Search for more papers by this authorCorresponding Author
Haiying Zhao
Inner Mongolia Key Laboratory of Synthesis and Application of Organic Functional Molecules, College of Chemistry and Chemical Engineering, Inner Mongolia University, Hohhot, Inner Mongolia, 010021 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Juntao Ye
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, Shanghai, 200240 China
E-mail: [email protected]; [email protected]Search for more papers by this authorXiao Yang
Inner Mongolia Key Laboratory of Synthesis and Application of Organic Functional Molecules, College of Chemistry and Chemical Engineering, Inner Mongolia University, Hohhot, Inner Mongolia, 010021 China
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, Shanghai, 200240 China
Search for more papers by this authorMengdi Ma
Inner Mongolia Key Laboratory of Synthesis and Application of Organic Functional Molecules, College of Chemistry and Chemical Engineering, Inner Mongolia University, Hohhot, Inner Mongolia, 010021 China
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, Shanghai, 200240 China
Search for more papers by this authorMeichen Xu
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, Shanghai, 200240 China
Search for more papers by this authorYubing Pang
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, Shanghai, 200240 China
Search for more papers by this authorCorresponding Author
Haiying Zhao
Inner Mongolia Key Laboratory of Synthesis and Application of Organic Functional Molecules, College of Chemistry and Chemical Engineering, Inner Mongolia University, Hohhot, Inner Mongolia, 010021 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Juntao Ye
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, Shanghai, 200240 China
E-mail: [email protected]; [email protected]Search for more papers by this author† Dedicated to the Special Issue of Emerging Investigators in 2024.
Comprehensive Summary
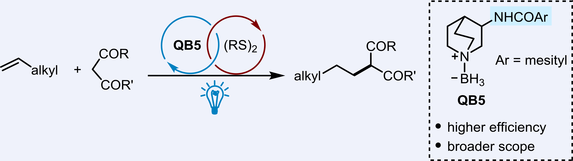
An amide-substituted quinuclidine-borane has been identified as a more efficient hydridic hydrogen atom transfer (HAT) catalyst for the hydroalkylation of unactivated olefins under visible-light irradiation. 1H NMR titration experiments reveal that the amide moiety of the quinuclidine-borane catalyst forms stronger hydrogen bonds with the carbonyl substrates, thereby improving the reaction yields. Furthermore, it was found that the reaction yields correlate well with the association constant between the quinuclidine-borane catalyst and the carbonyl substrate. A scale-up reaction using a continuous-flow photoreactor has also been demonstrated.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202400725-sup-0001-supinfo.pdfPDF document, 8.9 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For selected reviews, see: (a) Mayer, J. M. Understanding Hydrogen Atom Transfer: From Bond Strengths to Marcus Theory. Acc. Chem. Res. 2011, 44, 36–46;
(b) Capaldo, L.; Ravelli, D. Hydrogen Atom Transfer (HAT): A Versatile Strategy for Substrate Activation in Photocatalyzed Organic Synthesis. Eur. J. Org. Chem. 2017, 2017, 2056–2071;
(c) Chu, J. C. K.; Rovis, T. Complementary Strategies for Directed C(sp3)–H Functionalization: A Comparison of Transition-Metal- Catalyzed Activation, Hydrogen Atom Transfer, and Carbene/Nitrene Transfer. Angew. Chem. Int. Ed. 2018, 57, 62–101;
(d) Stateman, L. M.; Nakafuku, K. M.; Nagib, D. A. Remote C–H Functionalization via Selective Hydrogen Atom Transfer. Synthesis 2018, 50, 1569–1586;
(e) Zhang, J.; Liu, D.; Chen, Y. Investigations on the 1,2-Hydrogen Atom Transfer Reactivity of Alkoxyl Radicals under Visible-Light-Induced Reaction Conditions. Synlett 2020, 32, 356–361;
(f) Cao, H.; Tang, X.; Tang, H.; Yuan, Y.; Wu, J. Photoinduced Intermolecular Hydrogen Atom Transfer Reactions in Organic Synthesis. Chem Catal. 2021, 1, 523–598;
(g) Capaldo, L.; Ravelli, D.; Fagnoni, M. Direct Photocatalyzed Hydrogen Atom Transfer (HAT) for Aliphatic C–H Bonds Elaboration. Chem. Rev. 2021, 122, 1875–1924;
(h) Cheng, S.; Li, Q.; Cheng, X.; Lin, Y. M.; Gong, L. Recent Advances in Asymmetric Transformations of Unactivated Alkanes and Cycloalkanes through Direct C–H Functionalization. Chin. J. Chem. 2022, 40, 2825–2837;
(i) Galeotti, M.; Salamone, M.; Bietti, M. Electronic Control over Site-Selectivity in Hydrogen Atom Transfer (HAT) Based C(sp3)–H Functionalization Promoted by Electrophilic Reagents. Chem. Soc. Rev. 2022, 51, 2171–2223;
(j) Golden, D. L.; Suh, S.-E.; Stahl, S. S. Radical C(sp3)–H Functionalization and Cross-Coupling Reactions. Nat. Rev. Chem. 2022, 6, 405–427;
(k) Kim, M.; Koo, Y.; Hong, S. N-Functionalized Pyridinium Salts: A New Chapter for Site-Selective Pyridine C–H Functionalization via Radical-Based Processes under Visible Light Irradiation. Acc. Chem. Res. 2022, 55, 3043–3056;
(l) Murray, P. R. D.; Cox, J. H.; Chiappini, N. D.; Roos, C. B.; McLoughlin, E. A.; Hejna, B. G.; Nguyen, S. T.; Ripberger, H. H.; Ganley, J. M.; Tsui, E.; Shin, N. Y.; Koronkiewicz, B.; Qiu, G.; Knowles, R. R. Photochemical and Electrochemical Applications of Proton-Coupled Electron Transfer in Organic Synthesis. Chem. Rev. 2022, 122, 2017–2291;
(m) Wu, X.; Zhu, C. Combination of Radical Functional Group Migration (FGM) and Hydrogen Atom Transfer (HAT). Trends Chem. 2022, 4, 580–583;
(n) Zhang, Z.; Chen, P.; Liu, G. Copper-Catalyzed Radical Relay in C(sp3)–H Functionalization. Chem. Soc. Rev. 2022, 51, 1640–1658;
(o) Chang, L.; Wang, S.; An, Q.; Liu, L.; Wang, H.; Li, Y.; Feng, K.; Zuo, Z. Resurgence and Advancement of Photochemical Hydrogen Atom Transfer Processes in Selective Alkane Functionalizations. Chem. Sci. 2023, 14, 6841–6859;
(p) Yang, C.; Arora, S.; Maldonado, S.; Pratt, D. A.; Stephenson, C. R. J. The Design of PINO-Like Hydrogen-Atom-Transfer Catalysts. Nat. Rev. Chem. 2023, 7, 653–666;
(q) Zhang, J.; Rueping, M. Metallaphotoredox Catalysis for sp3 C–H Functionalizations through Hydrogen Atom Transfer (HAT). Chem. Soc. Rev. 2023, 52, 4099–4120;
(r) Li, T.; Li, J.; Huo, H. Nucleophilic Radicals as Hydrogen Atom Abstractors in C(sp3)–H Functionalization Reactions. Chin. J. Chem. 2023, 41, 544–547;
(s) Ang, H. T.; Miao, Y.; Ravelli, D.; Wu, J. Pyridine N-Oxides as Hydrogen Atom Transfer Reagents for Site-Selective Photoinduced C(sp3)–H Functionalization. Nat. Synth. 2024, 3, 568–575;
10.1038/s44160-024-00528-2 Google Scholar(t) Xu, G.-Q.; Wang, W.-D.; Xu, P.-F. Photocatalyzed Enantioselective Functionalization of C(sp3)–H Bonds. J. Am. Chem. Soc. 2024, 146, 1209–1223.
- 2For selected reviews, see: (a) Crespi, S.; Fagnoni, M. Generation of Alkyl Radicals: From the Tyranny of Tin to the Photon Democracy. Chem. Rev. 2020, 120, 9790–9833; (b) Gant Kanegusuku, A. L.; Roizen, J. L. Recent Advances in Photoredox-Mediated Radical Conjugate Addition Reactions: An Expanding Toolkit for the Giese Reaction. Angew. Chem. Int. Ed. 2021, 60, 21116–21149; (c) Corcé, V.; Ollivier, C.; Fensterbank, L. Boron, Silicon, Nitrogen and Sulfur-Based Contemporary Precursors for the Generation of Alkyl Radicals by Single Electron Transfer and Their Synthetic Utilization. Chem. Soc. Rev. 2022, 51, 1470–1510.
- 3For selected reviews, see: (a) Lenardon, G. V. A.; Nicchio, L.; Fagnoni, M. Photogenerated Electrophilic Radicals for the Umpolung of Enolate Chemistry. J. Photochem. Photobiol., C 2021, 46, 100387;
(b) O'Brien, T. E.; Morris, A. O.; Villela, L. F.; Barriault, L. Synthetic Applications of Photochemically Generated Radicals from Protic C(sp3)−H Bonds. ChemCatChem 2023, 15, e202300989;
10.1002/cctc.202300989 Google Scholar(c) Yamashita, Y.; Kobayashi, S. Efficient Radical-Mediated Intermolecular α-Alkylation Reactions of Carbonyl Compounds with Nonactivated Alkenes. Chem. Asian J. 2024, 19, e202400319; For selected examples, see: (d) De Vleeschouwer, F.; Van Speybroeck, V.; Waroquier, M.; Geerlings, P.; De Proft, F. Electrophilicity and Nucleophilicity Index for Radicals. Org. Lett. 2007, 9, 2721–2724; (e) Li, Z.; Xiao, Y.; Liu, Z. Q. A Radical Anti-Markovnikov Addition of Alkyl Nitriles to Simple Alkenes via Selective sp3 C−H Bond Functionalization. Chem. Commun. 2015, 51, 9969–9971; (f) Fang, J.; Dong, W. L.; Xu, G. Q.; Xu, P. F. Photocatalyzed Metal-Free Alkylheteroarylation of Unactivated Olefins via Direct Acidic C(sp3)−H Bond Activation. Org. Lett. 2019, 21, 4480–4485; (g) Poudel, D. P.; Pokhrel, A.; Tak, R. K.; Shankar, M.; Giri, R. Photosensitized O2 Enables Intermolecular Alkene Cyclopropanation by Active Methylene Compounds. Science 2023, 381, 545–553.
- 4For selected reviews, see: (a) Tedder, J. M. Which Factors Determine the Reactivity and Regioselectivity of Free Radical Substitution and Addition Reactions? Angew. Chem. Int. Ed. Engl. 1982, 21, 401–410; (b) Roberts, B. P. Polarity-Reversal Catalysis of Hydrogen-Atom Abstraction Reactions: Concepts and Applications in Organic Chemistry. Chem. Soc. Rev. 1999, 28, 25–35; (c) Parsaee, F.; Senarathna, M. C.; Kannangara, P. B.; Alexander, S. N.; Arche, P. D. E.; Welin, E. R. Radical Philicity and Its Role in Selective Organic Transformations. Nat. Rev. Chem. 2021, 5, 486–499; (d) Ruffoni, A.; Mykura, R. C.; Bietti, M.; Leonori, D. The Interplay of Polar Effects in Controlling the Selectivity of Radical Reactions. Nat. Synth. 2022, 1, 682–695.
- 5For selected examples, see: (a) Wallentin, C. J.; Nguyen, J. D.; Finkbeiner, P.; Stephenson, C. R. Visible Light-Mediated Atom Transfer Radical Addition via Oxidative and Reductive Quenching of Photocatalysts. J. Am. Chem. Soc. 2012, 134, 8875–8884;
(b) Wei, X.-J.; Yang, D.-T.; Wang, L.; Song, T.; Wu, L.-Z.; Liu, Q. A Novel Intermolecular Synthesis of γ-Lactones via Visible-Light Photoredox Catalysis. Org. Lett. 2013, 15, 6054–6057;
(c) Arceo, E.; Montroni, E.; Melchiorre, P. Photo-Organocatalysis of Atom-Transfer Radical Additions to Alkenes. Angew. Chem. Int. Ed. 2014, 53, 12064–12068;
(d) Uraguchi, D.; Tsuchiya, Y.; Ohtani, T.; Enomoto, T.; Masaoka, S.; Yokogawa, D.; Ooi, T. Unveiling Latent Photoreactivity of Imines. Angew. Chem. Int. Ed. 2020, 59, 3665–3670;
(e) Singh, H.; Tak, R. K.; Poudel, D. P.; Giri, R. Catalytic Photoredox Carbobromination of Unactivated Alkenes with α-Bromocarbonyls via the Mechanistically Distinct Radical-Addition Radical-Pairing Pathway. ACS Catal. 2024, 6001–6008;
10.1021/acscatal.4c00955 Google Scholar(f) Fischer, D. M.; Freis, M.; Amberg, W. M.; Lindner, H.; Carreira, E. M. Organophotocatalytic Carbo-Heterofunctionalization of Unactivated Olefins with Pendant Nucleophiles. Chem. Sci. 2023, 14, 7256–7261; (g) Fischer, D. M.; Lindner, H.; Amberg, W. M.; Carreira, E. M. Intermolecular Organophotocatalytic Cyclopropanation of Unactivated Olefins. J. Am. Chem. Soc. 2023, 145, 774–780; (h) Huang, W.; Chen, W.; Wang, G.; Li, J.; Cheng, X.; Li, G. Thiyl-Radical-Catalyzed Photoreductive Hydrodifluoroacetamidation of Alkenes with Hantzsch Ester as a Multifunctional Reagent. ACS Catal. 2016, 6, 7471–7474; (i) Lin, Q. Y.; Xu, X. H.; Zhang, K.; Qing, F. L. Visible-Light-Induced Hydrodifluoromethylation of Alkenes with a Bromodifluoromethylphosphonium Bromide. Angew. Chem. Int. Ed. 2016, 55, 1479–83; (j) Wang, H.; Jui, N. T. Catalytic Defluoroalkylation of Trifluoromethylaromatics with Unactivated Alkenes. J. Am. Chem. Soc. 2018, 140, 163–166; (k) Su, Y. L.; Liu, G. X.; Liu, J. W.; Tram, L.; Qiu, H.; Doyle, M. P. Radical-Mediated Strategies for the Functionalization of Alkenes with Diazo Compounds. J. Am. Chem. Soc. 2020, 142, 13846–13855.
- 6For a review, see: (a) Mondal, M.; Bora, U. Recent Advances in Manganese(III) Acetate Mediated Organic Synthesis. RSC Adv. 2013, 3, 18716–18754; For selected examples, see: (b) Ohashi, M.; Nakatani, K.; Maeda, H.; Mizuno, K. Photochemical Monoalkylation of Propanedinitrile by Electron-Rich Alkenes. Org. Lett. 2008, 10, 2741–2743; (c) Ohashi, M.; Nakatani, K.; Maeda, H.; Mizuno, K. Selective Photochemical Monoalkylation of Active Methylene Compounds by Alkenes. A Green Pathway for Carbon–Carbon Bond Formation. J. Photochem. Photobiol., A 2010, 214, 161–170; (d) Baś, S.; Yamashita, Y.; Kobayashi, S. Development of Brønsted Base–Photocatalyst Hybrid Systems for Highly Efficient C–C Bond Formation Reactions of Malonates with Styrenes. ACS Catal. 2020, 10, 10546–10550; (e) Katta, N.; Zhao, Q. Q.; Mandal, T.; Reiser, O. Divergent and Synergistic Photocatalysis: Hydro- and Oxoalkylation of Vinyl Arenes for the Stereoselective Synthesis of Cyclopentanols via a Formal [4+1]-Annulation of 1,3-Dicarbonyls. ACS Catal. 2022, 12, 14398–14407; (f) Yamashita, Y.; Ogasawara, Y.; Banik, T.; Kobayashi, S. Photoinduced Efficient Catalytic α-Alkylation Reactions of Active Methylene and Methine Compounds with Nonactivated Alkenes. J. Am. Chem. Soc. 2023, 145, 23160–23166.
- 7For selected examples, see: (a) Paul, V.; Roberts, B. P. Homolytic Reactions of Ligated Boranes. Part 8. Electron Spin Resonance Studies of Radicals Derived from Ligated Alkylboranes. J. Chem. Soc., Perkin Trans. 2 1988, 1183−1193;
(b) Dang, H.-S.; Roberts, B. P. Amine-Boranes as Polarity Reversal Catalysts for Radical Chain 2,3-Epoxypropanation Reactions of Esters. Tetrahedron Lett. 1992, 33, 4621–4624;
(c) Dang, H.-S.; Roberts, B. P. Homolytic Reactions of Ligated Boranes. Part 17. Amine–Boranes as Polarity Reversal Catalysts for Radical Chain Reactions of Esters with Vinylic Epoxides and with Allylic tert-Butyl Peroxides. J. Chem. Soc., Perkin Trans. 1 1993, 891–898;
10.1039/P19930000891 Google Scholar(d) Cai, Y.; Dang, H.-S.; Roberts, B. P. Radical-Chain Functionalisation at C–H Centres Using an O-Oxiranylcarbinyl O-Silyl Ketene Acetal. Tetrahedron Lett. 2004, 45, 4405–4409.
- 8For selected examples, see: (a) Ueng, S. H.; Brahmi, M. M.; Derat, E.; Fensterbank, L.; Lacote, E.; Malacria, M.; Curran, D. P. Complexes of Borane and N-Heterocyclic Carbenes: A New Class of Radical Hydrogen Atom Donor. J. Am. Chem. Soc. 2008, 130, 10082–10083; (b) Pan, X.; Lacote, E.; Lalevee, J.; Curran, D. P. Polarity Reversal Catalysis in Radical Reductions of Halides by N-Heterocyclic Carbene Boranes. J. Am. Chem. Soc. 2012, 134, 5669–5674; (c) Pan, X.; Vallet, A.-L.; Schweizer, S.; Dahbi, K.; Delpech, B.; Blanchard, N.; Graff, B.; Geib, S. J.; Curran, D. P.; Lalevée, J.; Lacôte, E. Mechanistic and Preparative Studies of Radical Chain Homolytic Substitution Reactions of N-Heterocyclic Carbene Boranes and Disulfides. J. Am. Chem. Soc. 2013, 135, 10484–10491; (d) Dai, W.; Geib, S. J.; Curran, D. P. Facile Synthesis of α-N-Heterocyclic Carbene-Boryl Ketones from N-Heterocyclic Carbene-Boranes and Alkenyl Triflates. J. Am. Chem. Soc. 2019, 141, 12355–12361; (e) Dai, W.; Geib, S. J.; Curran, D. P. 1,4-Hydroboration Reactions of Electron-Poor Aromatic Rings by N-Heterocyclic Carbene Boranes. J. Am. Chem. Soc. 2020, 142, 6261–6267.
- 9For selected reviews, see: (a) Jin, J.; Xia, H.; Zhang, F.; Wang, Y.-F. Lewis-Base Boryl Radicals Enabled Borylation, Radical Catalysis and Reduction Reactions. Chin. J. Org. Chem. 2020, 40, 2185–2194; (b) Peng, T. Y.; Zhang, F. L.; Wang, Y.-F. Lewis Base-Boryl Radicals Enabled Borylation Reactions and Selective Activation of Carbon-Heteroatom Bonds. Acc. Chem. Res. 2023, 56, 169–186; For selected examples, see: (c) Ren, S.-C.; Zhang, F.-L.; Qi, J.; Huang, Y.-S.; Xu, A.-Q.; Yan, H.-Y.; Wang, Y.-F. Radical Borylation/Cyclization Cascade of 1,6-Enynes for the Synthesis of Boron-Handled Hetero- and Carbocycles. J. Am. Chem. Soc. 2017, 139, 6050–6053; (d) Qi, J.; Zhang, F. L.; Jin, J. K.; Zhao, Q.; Li, B.; Liu, L. X.; Wang, Y.-F. New Radical Borylation Pathways for Organoboron Synthesis Enabled by Photoredox Catalysis. Angew. Chem. Int. Ed. 2020, 59, 12876–12884; (e) Yu, Y.-J.; Zhang, F.-L.; Peng, T.-Y.; Wang, C.-L.; Cheng, J.; Chen, C.; Houk, K. N.; Wang, Y.-F. Sequential C–F Bond Functionalizations of Trifluoroacetamides and Acetates via Spin-Center Shifts. Science 2021, 371, 1232–1240; (f) Wang, C.-L.; Wang, J.; Jin, J.-K.; Li, B.; Phang, Y. L.; Zhang, F.-L.; Ye, T.; Xia, H.-M.; Hui, L.-W.; Su, J.-H.; Fu, Y.; Wang, Y.-F. Boryl Radical Catalysis Enables Asymmetric Radical Cycloisomerization Reactions. Science 2023, 382, 1056–1065; (g) Wang, J.; Lin Phang, Y.; Yu, Y. J.; Liu, N. N.; Xie, Q.; Zhang, F. L.; Jin, J. K.; Wang, Y.-F. Boryl Radical as a Catalyst in Enabling Intra- and Intermolecular Cascade Radical Cyclization Reactions: Construction of Polycyclic Molecules. Angew. Chem. Int. Ed. 2024, 63, e202405863.
- 10For selected reviews, see: (a) Taniguchi, T. Advances in Chemistry of N-Heterocyclic Carbene Boryl Radicals. Chem. Soc. Rev. 2021, 50, 8995–9021;
(b) Zhao, Q.; Dewhurst, R. D.; Braunschweig, H.; Chen, X. A New Perspective on Borane Chemistry: The Nucleophilicity of the B−H Bonding Pair Electrons. Angew. Chem. Int. Ed. 2019, 58, 3268–3278;
(c) Tian, Y.-M.; Guo, X.-N.; Braunschweig, H.; Radius, U.; Marder, T. B. Photoinduced Borylation for the Synthesis of Organoboron Compounds. Chem. Rev. 2021, 121, 3561–3597;
(d) Capaldo, L.; Noël, T.; Ravelli, D. Photocatalytic Generation of Ligated Boryl Radicals from Tertiary Amine-Borane Complexes: An Emerging Tool in Organic Synthesis. Chem Catal. 2022, 2, 957–966;
10.1016/j.checat.2022.03.005 Google Scholar(e) Du, Y.; Dong, J.; Xu, C.; Pan, X. Organoboron Chemistry Towards Controlled and Precise Polymer Synthesis. Sci. China Chem. 2023, 66, 3467–3483; For selected examples, see: (f) Lu, D.; Wu, C.; Li, P. Synergistic Effects of Lewis Bases and Substituents on the Electronic Structure and Reactivity of Boryl Radicals. Chem.-Eur. J. 2014, 20, 1630–1637; (g) Zhou, N.; Yuan, X. A.; Zhao, Y.; Xie, J.; Zhu, C. Synergistic Photoredox Catalysis and Organocatalysis for Inverse Hydroboration of Imines. Angew. Chem. Int. Ed. 2018, 57, 3990–3994; (h) Xia, P. J.; Song, D.; Ye, Z. P.; Hu, Y. Z.; Xiao, J. A.; Xiang, H. Y.; Chen, X. Q.; Yang, H. Photoinduced Single-Electron Transfer as an Enabling Principle in the Radical Borylation of Alkenes with Nhc-Borane. Angew. Chem. Int. Ed. 2020, 59, 6706–6710; (i) Xu, W.; Jiang, H.; Leng, J.; Ong, H. W.; Wu, J. Visible-Light-Induced Selective Defluoroborylation of Polyfluoroarenes, Gem-Difluoroalkenes, and Trifluoromethylalkenes. Angew. Chem. Int. Ed. 2020, 59, 4009–4016; (j) Kim, J. H.; Constantin, T.; Simonetti, M.; Llaveria, J.; Sheikh, N. S.; Leonori, D. A Radical Approach for the Selective C–H Borylation of Azines. Nature 2021, 595, 677–683; (k) Choi, W.; Kim, M.; Lee, K.; Park, S.; Hong, S. C4-Selective C–H Borylation of Pyridinium Derivatives Driven by Electron Donor–Acceptor Complexes. Org. Lett. 2022, 24, 9452–9457; (l) Wang, Z.; Chen, J.; Lin, Z.; Quan, Y. Photoinduced Dehydrogenative Borylation Via Dihydrogen Bond Bridged Electron Donor and Acceptor Complexes. Chem.-Eur. J. 2022, e202203053; (m) Miao, Y.-Q.; Pan, Q.-J.; Kang, J.-X.; Dai, X.; Liu, Z.; Chen, X. A Green and Facile Photochemical Thiolate-Catalyzed Strategy for Borylation of Aryl Fluorides with Nhc–Borane. Org. Chem. Front. 2024, 11, 1462–1468.
- 11For a recent book, see: Stephenson, C. R. J.; Yoon, T. P.; MacMillan, D. W. C. Visible Light Photocatalysis in Organic Chemistry, Wiley-VCH, Weinheim, 2018.
10.1002/9783527674145 Google Scholar
- 12For selected reviews, see: (a) Romero, N. A.; Nicewicz, D. A. Organic Photoredox Catalysis. Chem. Rev. 2016, 116, 10075–10166; (b) Marzo, L.; Pagire, S. K.; Reiser, O.; Konig, B. Visible-Light Photocatalysis: Does It Make a Difference in Organic Synthesis? Angew. Chem. Int. Ed. 2018, 57, 10034–10072; (c) Silvi, M.; Melchiorre, P. Enhancing the Potential of Enantioselective Organocatalysis with Light. Nature 2018, 554, 41–49; (d) Chen, Y.; Lu, L.-Q.; Yu, D.-G.; Zhu, C.-J.; Xiao, W.-J. Visible Light-Driven Organic Photochemical Synthesis in China. Sci. China Chem. 2019, 62, 24–57; (e) Wang, H.; Gao, X.; Lv, Z.; Abdelilah, T.; Lei, A. Recent Advances in Oxidative R1-H/R2-H Cross-Coupling with Hydrogen Evolution via Photo-/Electrochemistry. Chem. Rev. 2019, 119, 6769–6787; (f) Bell, J. D.; Murphy, J. A. Recent Advances in Visible Light-Activated Radical Coupling Reactions Triggered by (I) Ruthenium, (II) Iridium and (III) Organic Photoredox Agents. Chem. Soc. Rev. 2021, 50, 9540–9685; (g) Vega-Penaloza, A.; Mateos, J.; Companyo, X.; Escudero-Casao, M.; Dell'Amico, L. A Rational Approach to Organo-Photocatalysis: Novel Designs and Structure-Property Relationships. Angew. Chem. Int. Ed. 2021, 60, 1082–1097; (h) Yu, X. Y.; Chen, J. R.; Xiao, W. J. Visible Light-Driven Radical-Mediated C–C Bond Cleavage/Functionalization in Organic Synthesis. Chem. Rev. 2021, 121, 506–561; (i) Buglioni, L.; Raymenants, F.; Slattery, A.; Zondag, S. D. A.; Noël, T. Technological Innovations in Photochemistry for Organic Synthesis: Flow Chemistry, High- Throughput Experimentation, Scale-up, and Photoelectrochemistry. Chem. Rev. 2022, 122, 2752–2906; (j) Chang, L.; An, Q.; Duan, L.; Feng, K.; Zuo, Z. Alkoxy Radicals See the Light: New Paradigms of Photochemical Synthesis. Chem. Rev. 2022, 122, 2429–2486; (k) Großkopf, J.; Kratz, T.; Rigotti, T.; Bach, T. Enantioselective Photochemical Reactions Enabled by Triplet Energy Transfer. Chem. Rev. 2022, 122, 1626–1653; (l) Meng, S.-L.; Ye, C.; Li, X.-B.; Tung, C.-H.; Wu, L.-Z. Photochemistry Journey to Multielectron and Multiproton Chemical Transformation. J. Am. Chem. Soc. 2022, 144, 16219–16231; (m) Tay, N. E. S.; Lehnherr, D.; Rovis, T. Photons or Electrons? A Critical Comparison of Electrochemistry and Photoredox Catalysis for Organic Synthesis. Chem. Rev. 2022, 122, 2487–2649; (n) Nevesely, T.; Wienhold, M.; Molloy, J. J.; Gilmour, R. Advances in the E → Z Isomerization of Alkenes Using Small Molecule Photocatalysts. Chem. Rev. 2022, 122, 2650–2694; (o) Bellotti, P.; Huang, H. M.; Faber, T.; Glorius, F. Photocatalytic Late-Stage C–H Functionalization. Chem. Rev. 2023, 123, 4237–4352; (p) Goti, G.; Manal, K.; Sivaguru, J.; Dell’Amico, L. The Impact of UV Light on Synthetic Photochemistry and Photocatalysis. Nat. Chem. 2024, 16, 684–692.
- 13 Lei, G.; Xu, M.; Chang, R.; Funes-Ardoiz, I.; Ye, J. Hydroalkylation of Unactivated Olefins via Visible-Light-Driven Dual Hydrogen Atom Transfer Catalysis. J. Am. Chem. Soc. 2021, 143, 11251–11261.
- 14For selected reviews, see: (a) Lv, X.; Xu, H.; Yin, Y.; Zhao, X.; Jiang, Z. Visible Light-Driven Cooperative DPZ and Chiral Hydrogen-Bonding Catalysis. Chin. J. Chem. 2020, 38, 1480–1488; (b) Proctor, R. S. J.; Colgan, A. C.; Phipps, R. J. Exploiting Attractive Non-Covalent Interactions for the Enantioselective Catalysis of Reactions Involving Radical Intermediates. Nat. Chem. 2020, 12, 990–1004; (c) Sempere, Y.; Morgenstern, M.; Bach, T.; Plaza, M. Reactivity and Selectivity Modulation within a Molecular Assembly: Recent Examples from Photochemistry. Photochem. Photobiol. Sci. 2021, 21, 719–737.
- 15For selected reviews, see: (a) Fielding, L. Determination of Association Constants (Ka) from Solution Nmr Data. Tetrahedron 2000, 56, 6151–6170; (b) Thordarson, P. Determining Association Constants from Titration Experiments in Supramolecular Chemistry. Chem. Soc. Rev. 2011, 40, 1305–1323; For a recent example, see: (c) Plaza, M.; Großkopf, J.; Breitenlechner, S.; Bannwarth, C.; Bach, T. Photochemical Deracemization of Primary Allene Amides by Triplet Energy Transfer: A Combined Synthetic and Theoretical Study. J. Am. Chem. Soc. 2021, 143, 11209–11217.
- 16 Ladouceur, S.; Fortin, D.; Zysman-Colman, E. Enhanced Luminescent Iridium(III) Complexes Bearing Aryltriazole Cyclometallated Ligands. Inorg. Chem. 2011, 50, 11514–11526.




