Transition-Metal-Mediated Fluoroalkylation of Carbon Electrophiles through Cross-Electrophile Couplings
Yun-Cheng Luo
School of Chemistry and Material Sciences, Hangzhou Institute for Advanced Study, University of Chinese Academy of Sciences, 1 Sub-lane Xiangshan, Hangzhou, Zhejiang, 310024 China
Search for more papers by this authorCorresponding Author
Xingang Zhang
School of Chemistry and Material Sciences, Hangzhou Institute for Advanced Study, University of Chinese Academy of Sciences, 1 Sub-lane Xiangshan, Hangzhou, Zhejiang, 310024 China
Key Laboratory of Fluorine and Nitrogen Chemistry and Advanced Materials (Chinese Academy of Sciences), Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
E-mail: [email protected]Search for more papers by this authorYun-Cheng Luo
School of Chemistry and Material Sciences, Hangzhou Institute for Advanced Study, University of Chinese Academy of Sciences, 1 Sub-lane Xiangshan, Hangzhou, Zhejiang, 310024 China
Search for more papers by this authorCorresponding Author
Xingang Zhang
School of Chemistry and Material Sciences, Hangzhou Institute for Advanced Study, University of Chinese Academy of Sciences, 1 Sub-lane Xiangshan, Hangzhou, Zhejiang, 310024 China
Key Laboratory of Fluorine and Nitrogen Chemistry and Advanced Materials (Chinese Academy of Sciences), Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
E-mail: [email protected]Search for more papers by this authorComprehensive Summary
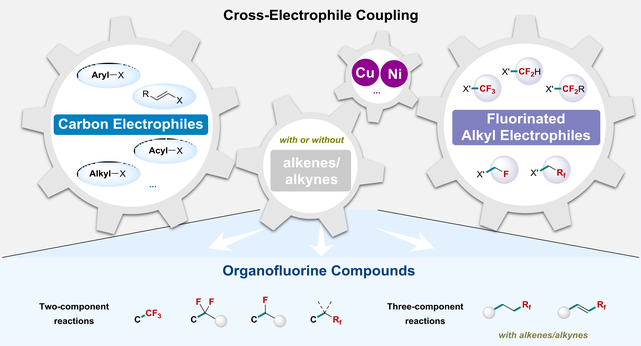
Organofluorine compounds have attracted substantial interest in life and materials sciences due to the unique properties of fluorine atom(s) that often change the physicochemical and biological properties of organic molecules. Transition-metal-mediated cross-electrophile coupling between carbon electrophiles and fluoroalkyl electrophiles has emerged as a straightforward and efficient route for the synthesis of a wide range of fluoroalkylated compounds because of its synthetic convenience without the tedious synthesis of organometallic reagents. Moreover, alkenes or alkynes-involved three-component cross-electrophile couplings provide rapid and effective access to carbonfunctionalized fluoroalkylated alkanes and alkenes. Herein, we comprehensively summarize the transition-metal-mediated reductive fluoroalkylation of diverse carbon electrophiles through a historical perspective, including trifluoromethylation, difluoroalkylation, monofluoroalkylation, and so on. Different transition metals (Cu, Ni, etc.) and strategies are discussed, in which nickel-catalyzed reductive fluoroalkylation reactions represent an attractive and efficient synthetic route to site-selectively access organofluorine compounds.
Key Scientists
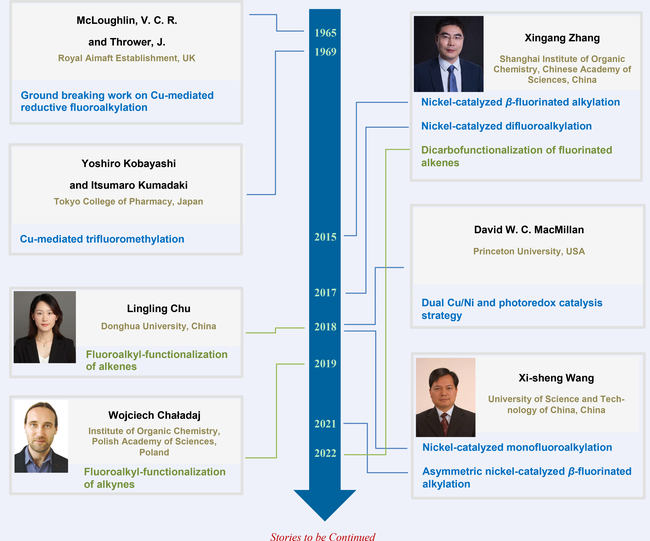
As early as 1965, McLoughlin and Thrower finished the first stoichiometric copper-mediated fluoroalkylation of aromatic iodides with fluoroalkyl iodides. However, excess aromatic iodides and elevated temperature were used for this method. In 1969, Kobayashi and Kumadaki reported studies on the copper-mediated trifluoromethylation of aromatic halides with excess trifluoromethyl iodide. After more than four decades, the Zhang group developed a nickel-catalyzed β-fluorinated alkylation of (hetero)aryl iodides with fluoroalkylated secondary alkyl bromides in 2015, and a nickel-catalyzed difluoromethylation of (hetero)aryl chlorides with chlorodifluoromethane ClCF2H in 2017. The Zhang group also developed enantioselective nickel-catalyzed reductive alkyl-arylation of 3,3,3-trifluoropropene with (hetero)aryl and tertiary alkyl iodides. In 2018, the MacMillan group developed a novel copper/photoredox dual catalytic system for the trifluoromethylation of aryl bromides or alkyl bromides with (S)-(trifluoromethyl) dimesitylsulfonium triflate in the presence of tris-(trimethylsilyl) silanol. They also developed a nickel/photoredox catalyzed difluoromethylation of aryl bromides in the presence of silane. During this time, the Wang group reported a nickel-catalyzed monofluoroalkylation of aryl halides with monofluoroalkyl halides. From 2021 to 2023, the same group further developed a series of enantioselective nickel-catalyzed trifluoroalkylation of aryl, alkenyl, and acyl halides. Moreover, nonfluorinated alkenes or alkynes could also be used in three-component cross-electrophile couplings. In 2018, the Chu group reported a nickel-catalyzed fluoroalkyl-acylation of alkenes with acyl chlorides and fluoroalkyl iodides. Later, they developed a nickel-catalyzed enantioselective fluoroalkyl-arylation of unactivated alkenes tethering with a pendant chelating group. In 2019, the Chaładaj group reported a palladium-catalyzed reductive perfluoroalkyl-arylation of alkynes with perfluoroalkyl and aryl iodides.
References
- 1 Hagmann, W. K. The Many Roles for Fluorine in Medicinal Chemistry. J. Med. Chem. 2008, 51, 4359–4369.
- 2 O'Hagan, D. Understanding Organofluorine Chemistry. An Introduction to the C-F Bond. Chem. Soc. Rev. 2008, 37, 308–319.
- 3 Ameduri, B. Fluoropolymers: The Right Material for the Right Applications. Chem. Eur. J. 2018, 24, 18830–18841.
- 4 Sicard, A. J.; Baker, R. T. Fluorocarbon Refrigerants and Their Syntheses: Past to Present. Chem. Rev. 2020, 120, 9164–9303.
- 5 Jaye, J. A.; Sletten, E. M. Recent Advances in the Preparation of Semifluorinated Polymers. Polym. Chem. 2021, 12, 6515–6526.
- 6 Lv, J.; Cheng, Y. Fluoropolymers in Biomedical Applications: State-of- the-Art and Future Perspectives. Chem. Soc. Rev. 2021, 50, 5435–5467.
- 7 Gillis, E. P.; Eastman, K. J.; Hill, M. D.; Donnelly, D. J.; Meanwell, N. A. Applications of Fluorine in Medicinal Chemistry. J. Med. Chem. 2015, 58, 8315–8359.
- 8 Meanwell, N. A. Fluorine and Fluorinated Motifs in the Design and Application of Bioisosteres for Drug Design. J. Med. Chem. 2018, 61, 5822–5880.
- 9 Ogawa, Y.; Tokunaga, E.; Kobayashi, O.; Hirai, K.; Shibata, N. Current Contributions of Organofluorine Compounds to the Agrochemical Industry. iScience 2020, 23, 101467.
- 10 Zhang, C.; Yan, K.; Fu, C.; Peng, H.; Hawker, C. J.; Whittaker, A. K. Biological Utility of Fluorinated Compounds: from Materials Design to Molecular Imaging, Therapeutics and Environmental Remediation. Chem. Rev. 2022, 122, 167–208.
- 11 Liang, T.; Neumann, C. N.; Ritter, T. Introduction of Fluorine and Fluorine-Containing Functional Groups. Angew. Chem. Int. Ed. 2013, 52, 8214–8264.
- 12 Ahrens, T.; Kohlmann, J.; Ahrens, M.; Braun, T. Functionalization of Fluorinated Molecules by Transition-Metal-Mediated C-F Bond Activation to Access Fluorinated Building Blocks. Chem. Rev. 2015, 115, 931–972.
- 13 Champagne, P. A.; Desroches, J.; Hamel, J.-D.; Vandamme, M.; Paquin, J.-F. Monofluorination of Organic Compounds: 10 Years of Innovation. Chem. Rev. 2015, 115, 9073–9174.
- 14 Liu, X.; Xu, C.; Wang, M.; Liu, Q. Trifluoromethyltrimethylsilane: Nucleophilic Trifluoromethylation and Beyond. Chem. Rev. 2015, 115, 683–730.
- 15 Yang, X.; Wu, T.; Phipps, R. J.; Toste, F. D. Advances in Catalytic Enantioselective Fluorination, Mono-, Di-, and trifluoromethylation, and Trifluoromethylthiolation Reactions. Chem. Rev. 2015, 115, 826–870.
- 16 Fujita, T.; Fuchibe, K.; Ichikawa, J. Transition-Metal-Mediated and -Catalyzed C-F Bond Activation by Fluorine Elimination. Angew. Chem. Int. Ed. 2019, 58, 390–402.
- 17 Reichel, M.; Karaghiosoff, K. Reagents for Selective Fluoromethylation: A Challenge in Organofluorine Chemistry. Angew. Chem. Int. Ed. 2020, 59, 12268–12281.
- 18 Fu, W. C.; MacQueen, P. M.; Jamison, T. F. Continuous Flow Strategies for using Fluorinated Greenhouse Gases in Fluoroalkylations. Chem. Soc. Rev. 2021, 50, 7378–7394.
- 19 Feng, Z.; Xiao, Y.-L.; Zhang, X. Transition-Metal (Cu, Pd, Ni)-Catalyzed Difluoroalkylation via Cross-Coupling with Difluoroalkyl Halides. Acc. Chem. Res. 2018, 51, 2264–2278.
- 20 Carvalho, D. R.; Christian, A. H. Modern Approaches towards the Synthesis of Geminal Difluoroalkyl Groups. Org. Biomol. Chem. 2021, 19, 947–964.
- 21 Sap, J. B. I.; Meyer, C. F.; Straathof, N. J. W.; Iwumene, N.; Am Ende, C. W.; Trabanco, A. A.; Gouverneur, V. Late-Stage Difluoromethylation: Concepts, Developments and Perspective. Chem. Soc. Rev. 2021, 50, 8214–8247.
- 22 Zhou, W.; Pan, W.-J.; Chen, J.; Zhang, M.; Lin, J.-H.; Cao, W.; Xiao, J.-C. Transition-Metal Difluorocarbene Complexes. Chem. Commun. 2021, 57, 9316–9329.
- 23 Qing, F.-L.; Liu, X.-Y.; Ma, J.-A.; Shen, Q.; Song, Q.; Tang, P. A Fruitful Decade of Organofluorine Chemistry: New Reagents and Reactions. CCS Chem. 2022, 4, 2518–2549.
- 24 Everson, D. A.; Weix, D. J. Cross-Electrophile Coupling: Principles of Reactivity and Selectivity. J. Org. Chem. 2014, 79, 4793–4798.
- 25 Weix, D. J. Methods and Mechanisms for Cross-Electrophile Coupling of Csp2 Halides with Alkyl Electrophiles. Acc. Chem. Res. 2015, 48, 1767–1775.
- 26 Liu, J.; Ye, Y.; Sessler, J. L.; Gong, H. Cross-Electrophile Couplings of Activated and Sterically Hindered Halides and Alcohol Derivatives. Acc. Chem. Res. 2020, 53, 1833–1845.
- 27 Luo, Y.-C.; Xu, C.; Zhang, X. Nickel-Catalyzed Dicarbofunctionalization of Alkenes. Chin. J. Chem. 2020, 38, 1371–1394.
- 28 Burton, D. J.; Yang, Z.-Y. Fluorinated Organometallics: Perfluoroalkyl and Functionalized Perfluoroalkyl Organometallic Reagents in Organic Synthesis. Tetrahedron 1992, 48, 189–275.
- 29 Tomashenko, O. A.; Grushin, V. V. Aromatic Trifluoromethylation with Metal Complexes. Chem. Rev. 2011, 111, 4475–4521.
- 30 Cho, E. J.; Senecal, T. D.; Kinzel, T.; Zhang, Y.; Watson, D. A.; Buchwald, S. L. The Palladium-Catalyzed Trifluoromethylation of Aryl Chlorides. Science 2010, 328, 1679–1681.
- 31 Arlow, S. I.; Hartwig, J. F. Synthesis, Characterization, and Reactivity of Palladium Fluoroenolate Complexes. J. Am. Chem. Soc. 2017, 139, 16088–16091.
- 32 Ni, C.; Hu, J. The Unique Fluorine Effects in Organic Reactions: Recent Facts and Insights into Fluoroalkylations. Chem. Soc. Rev. 2016, 45, 5441–5454.
- 33 Kitagawa, O.; Taguchi, T.; Kobayashi, Y. (Methyl Difluoroacetate)copper Reagent. Remarkable Solvent Effect on the 19F-NMR Spectrum, Stability and Reactivity. Chem. Lett. 1989, 18, 389–392.
- 34 Sprague, L. G.; Burton, D. J.; Guneratne, R. D.; Bennett, W. E. Synthesis and Characterization of (E) and (Z)-1,2-Difluoroethenediylbisphosphonates. J. Fluor. Chem. 1990, 49, 75–85.
- 35 Yokomatsu, T.; Suemune, K.; Murano, T.; Shibuya, S. Synthesis of (α,α-Difluoroallyl)phosphonates from Alkenyl Halides or Acetylenes. J. Org. Chem. 1996, 61, 7207–7211.
- 36 Eujen, R.; Hoge, B.; Brauer, D. J. Synthesis and Properties of Donor- Free Bis(difluoromethyl) Cadmium, (CF2H)2Cd NMR Spectroscopic Detection and Structure of Tetrakis(difluoromethyl) Cuprate(III) and Related Compounds. J. Organomet. Chem. 1996, 519, 7–20.
- 37 Burton, D. J.; Hartgraves, G. A. The preparation of HCF2CdX and HCF2ZnX via direct insertion into the carbon halogen bond of CF2HY (Y=Br, I). J. Fluor. Chem. 2007, 128, 1198–1215.
- 38 Bour, J. R.; Kariofillis, S. K.; Sanford, M. S. Synthesis, Reactivity, and Catalytic Applications of Isolable (NHC)Cu(CHF2) Complexes. Organometallics 2017, 36, 1220–1223.
- 39
Kobayashi, Y.; Yamamoto, K.; Kumadaki, I. Trifluoromethylation of Aliphatic Halides with Trifluoromethyl Copper. Tetrahedron Lett. 1979, 20, 4071–4072.
10.1016/S0040-4039(01)86506-0 Google Scholar
- 40 Kobayashi, Y.; Yamamoto, K.; Asai, T.; Nakano, M.; Kumadaki, I. Studies on Organic Fluorine Compounds. Part 35. Trifluoromethylation of Pyrimidine- and Purine-Nucleosides with Trifluoromethyl- Copper Complex. J. Chem. Soc., Perkin Trans. 1 1980, 2755–2761.
- 41 Feng, Z.; Chen, F.; Zhang, X. Copper Catalyzed Cross-Coupling of Iodobenzoates with Bromozinc-difluorophosphonate. Org. Lett. 2012, 14, 1938–1941.
- 42 Zhao, Y.; Gao, B.; Ni, C.; Hu, J. Copper-Mediated Fluoroalkylation of Aryl Iodides Enables Facile Access to Diverse Fluorinated Compounds: The Important Role of the (2-Pyridyl)sulfonyl Group. Org. Lett. 2012, 14, 6080–6083.
- 43 Feng, Z.; Xiao, Y.-L.; Zhang, X. Copper-Catalyzed Cross-Coupling of Bromozinc-Difluoromethylphosphonate with Iodo/Bromo-Aryl Triazenes. Org. Chem. Front. 2014, 1, 113–116.
- 44 Kato, H.; Hirano, K.; Kurauchi, D.; Toriumi, N.; Uchiyama, M. Dialkylzinc-Mediated Cross-Coupling Reactions of Perfluoroalkyl and Perfluoroaryl Halides with Aryl Halides. Chem. Eur. J. 2015, 21, 3895–3900.
- 45 Evano, G.; Blanchard, N.; Toumi, M. Copper-Mediated Coupling Reactions and Their Applications in Natural Products and Designed Biomolecules Synthesis. Chem. Rev. 2008, 108, 3054–3131.
- 46 Sambiagio, C.; Marsden, S. P.; Blacker, A. J.; McGowan, P. C. Copper Catalysed Ullmann Type Chemistry: From Mechanistic Aspects to Modern Development. Chem. Soc. Rev. 2014, 43, 3525–3550.
- 47 Bhunia, S.; Pawar, G. G.; Kumar, S. V.; Jiang, Y.; Ma, D. Selected Copper-Based Reactions for C-N, C-O, C-S, and C-C Bond Formation. Angew. Chem. Int. Ed. 2017, 56, 16136–16179.
- 48 Cheng, L.-J.; Mankad, N. P. C-C and C-X Coupling Reactions of Unactivated Alkyl Electrophiles using Copper Catalysis. Chem. Soc. Rev. 2020, 49, 8036–8064.
- 49 Gu, Q.-S.; Li, Z.-L.; Liu, X.-Y. Copper(I)-Catalyzed Asymmetric Reactions Involving Radicals. Acc. Chem. Res. 2020, 53, 170–181.
- 50 Liu, R. Y.; Buchwald, S. L. CuH-Catalyzed Olefin Functionalization: From Hydroamination to Carbonyl Addition. Acc. Chem. Res. 2020, 53, 1229–1243.
- 51 Zhou, H.; Li, Z.-L.; Gu, Q.-S.; Liu, X.-Y. Ligand-Enabled Copper(I)-Catalyzed Asymmetric Radical C(sp3)–C Cross-Coupling Reactions. ACS Catal. 2021, 11, 7978–7986.
- 52 McLoughlin, V. C. R.; Thrower, J. British Patent 1156912 and U.S. Patent 3408411, 1966.
- 53 McLoughlin, V. C. R.; Thrower, J. A Route to Fluoroalkyl-Substituted Aromatic Compounds Involving Fluoroalkylcopper Intermediates. Tetrahedron 1969, 25, 5921–5940.
- 54 Critchley, J. P.; McLoughlin, V. C. R.; Thrower, J.; White, I. M. Perfluoroalkylene-linked Aromatic Polymers. Br. Polym. J. 1970, 2, 288–294.
- 55
Griffith, J. R.; O'Rear, J. G. Synthesis of 1,3-Bis[2-hydroxyhexafluoro- 2-propyl]-5-perfluoroalkylbenzenes. Synthesis 1974, 1974, 493–493.
10.1055/s-1974-23350 Google Scholar
- 56 Sepio, J.; Soulen, R. L. Perfluoroalkylation of Benzene Derivatives. An Improved Synthesis of 1,3-Bis(2-hydroxyhexafluoro-2-propyl)-5-(perfluoro-n-alkyl)benzenes. J. Fluor. Chem. 1984, 24, 61–74.
- 57 Burdon, J.; Coe, P. L.; Marsh, C. R.; Tatlow, J. C. Reactions of Organocopper Compounds with Halogeno-Olefins. Chem. Commun. 1967, 1259–1260.
- 58 Burdon, J.; Coe, P. L.; Marsh, C. R.; Tatlow, J. C. Reactions of Organocopper Compounds. Part I. J. Chem. Soc., Perkin Trans. 1 1972, 639–641.
- 59
Coe, P. L.; Milner, N. E.; Smith, J. A. Reactions of Perfluoroalkylcopper Compounds. Part V. The Preparation of Some Polyfluoroalkyl-Substituted Acids and Alcohols. J. Chem. Soc., Perkin Trans. 1 1975, 654–656.
10.1039/p19750000654 Google Scholar
- 60
Kobayashi, Y.; Kumadaki, I. Trifluoromethylation of Aromatic Compounds. Tetrahedron Lett. 1969, 10, 4095–4096.
10.1016/S0040-4039(01)88624-X Google Scholar
- 61 Kobayashi, Y.; Kumadaki, I.; Sato, S.; Hara, N.; Chikami, E. Studies on Organic Fluorine Compounds. VII. Trifluoromethylation of Aromatic Compounds. Chem. Pharm. Bull. 1970, 18, 2334–2339.
- 62 Kobayashi, Y.; Kumadaki, I. Studies on Organic Fluorine Compounds. Part 27. Abnormal Reactions in the Trifluoromethylation of Aromatic Compounds with Trifluoromethyl Iodide and Copper Powder. J. Chem. Soc., Perkin Trans. 1 1980, 661–664.
- 63 Kobayashi, Y.; Kumadaki, I.; Yamamoto, K. Simple Synthesis of Trifluoromethylated Pyrimidine Nucleosides. J. Chem. Soc., Chem. Commun. 1977, 536–537.
- 64
Clark, J. H.; McClinton, M. A.; Blade, R. J. The Direct Trifluoromethylation of Aryl Chlorides using Burton's Reagent. J. Chem. Soc., Chem. Commun. 1988, 638–639.
10.1039/c39880000638 Google Scholar
- 65 Clark, J. H.; McClinton, M. A.; Jones, C. W.; Landon, P.; Bishop, D.; Blade, R. J. The Effect of Charcoal on the Trifluoromethylation of Aryl Chlorides using Burton's Reagent. Tetrahedron Lett. 1989, 30, 2133–2136.
- 66 Clark, J. H.; Denness, J. E.; McClinton, M. A.; Wynd, A. J. The Trifluoromethylation of Chloroaromatics using the Copper-CF2Br2- Dialkylamide Reaction System. J. Fluor. Chem. 1990, 50, 411–426.
- 67 Zhang, C.-P.; Wang, Z.-L.; Chen, Q.-Y.; Zhang, C.-T.; Gu, Y.-C.; Xiao, J.-C. Copper-Mediated Trifluoromethylation of Heteroaromatic Compounds by Trifluoromethyl Sulfonium Salts. Angew. Chem. Int. Ed. 2011, 50, 1896–1900.
- 68 Liu, Y.; Shao, X.; Zhang, P.; Lu, L.; Shen, Q. Trifluoromethyl- Substituted Sulfonium Ylide: Rh-Catalyzed Carbenoid Addition to Trifluoromethylthioether. Org. Lett. 2015, 17, 2752–2755.
- 69 Dai, J.-J.; Fang, C.; Xiao, B.; Yi, J.; Xu, J.; Liu, Z.-J.; Lu, X.; Liu, L.; Fu, Y. Copper-promoted Sandmeyer Trifluoromethylation Reaction. J. Am. Chem. Soc. 2013, 135, 8436–8439.
- 70 Hong, J.; Huo, L.; Yang, Y.; Wang, G.; Zheng, C. Copper-Promoted One-Pot Trifluoromethylation of Aromatic Amines with Togni's Reagent. ChemistrySelect 2017, 2, 3716–3720.
- 71 Xue, J. H.; Li, Y.; Liu, Y.; Li, Q.; Wang, H. Site-Specific Deaminative Trifluoromethylation of Aliphatic Primary Amines. Angew. Chem. Int. Ed. 2024, 63, e202319030.
- 72 Le, C.; Chen, T. Q.; Liang, T.; Zhang, P.; MacMillan, D. W. C. A Radical Approach to the Copper Oxidative Addition Problem: Trifluoromethylation of Bromoarenes. Science 2018, 360, 1010–1014.
- 73 Chen, Y.; Ma, G.; Gong, H. Copper-Catalyzed Reductive Trifluoromethylation of Alkyl Iodides with Togni's Reagent. Org. Lett. 2018, 20, 4677–4680.
- 74 Kornfilt, D. J. P.; MacMillan, D. W. C. Copper-Catalyzed Trifluoromethylation of Alkyl Bromides. J. Am. Chem. Soc. 2019, 141, 6853–6858.
- 75 Taguchi, T.; Kitagawa, O.; Morikawa, T.; Nishiwaki, T.; Uehara, H.; Endo, H.; Kobayashi, Y. Synthesis of 2,2-difluoroesters by iododifluoroacetate-copper with organic halides. Tetrahedron Lett. 1986, 27, 6103–6106.
- 76 Sato, K.; Kawata, R.; Ama, F.; Omote, M.; Ando, A.; Kumadaki, I. Synthesis of Alkenyl- and Aryldifluoroacetate Using a Copper Complex from Ethyl Bromodifluoroacetate. Chem. Pharm. Bull. 1999, 47, 1013–1016.
- 77 Sato, K.; Omote, M.; Ando, A.; Kumadaki, I. Reactions of Ethyl Bromodifluoroacetate in the Presence of Copper Powder. J. Fluor. Chem. 2004, 125, 509–515.
- 78 Zhu, J.; Zhang, W.; Zhang, L.; Liu, J.; Zheng, J.; Hu, J. Copper-mediated Fluoroalkylation Reactions with Iododifluoroacetamides: Controlling the Selectivity among Cross-Coupling, Intramolecular Cyclization, and Homocoupling Reactions. J. Org. Chem. 2010, 75, 5505–5512.
- 79 Jiang, H.; Lu, W.; Yang, K.; Ma, G.; Xu, M.; Li, J.; Yao, J.; Wan, W.; Deng, H.; Wu, S.; Zhu, S.; Hao, J. Enhancement of Neighbouring- Group Participation in Cu0-Promoted Cross-Coupling gem-Difluoromethylenation of Aryl/Alkenyl Halides with 1,3-Azolic Difluoromethyl Bromides. Chem. Eur. J. 2014, 20, 10084–10092.
- 80 Xu, S.; Chen, H.-H.; Dai, J.-J.; Xu, H.-J. Copper-Promoted Reductive Coupling of Aryl Iodides with 1,1,1-Trifluoro-2-iodoethane. Org. Lett. 2014, 16, 2306–2309.
- 81 Gu, J.; Wang, X.; Xue, W.; Gong, H. Nickel-Catalyzed Reductive Coupling of Alkyl Halides with Other Electrophiles: Concept and Mechanistic Considerations. Org. Chem. Front. 2015, 2, 1411–1421.
- 82 Xue, W.; Jia, X.; Wang, X.; Tao, X.; Yin, Z.; Gong, H. Nickel-Catalyzed Formation of Quaternary Carbon Centers using Tertiary Alkyl Electrophiles. Chem. Soc. Rev. 2021, 50, 4162–4184.
- 83 Tasker, S. Z.; Standley, E. A.; Jamison, T. F. Recent Advances in Homogeneous Nickel Catalysis. Nature 2014, 509, 299–309.
- 84 Diccianni, J. B.; Diao, T. Mechanisms of Nickel-Catalyzed Cross-Coupling Reactions. Trends Chem. 2019, 1, 830–844.
- 85 Zhu, C.; Yue, H.; Chu, L.; Rueping, M. Recent Advances in Photoredox and Nickel Dual-Catalyzed Cascade Reactions: Pushing the Boundaries of Complexity. Chem. Sci. 2020, 11, 4051–4064.
- 86 Bour, J. R.; Camasso, N. M.; Sanford, M. S. Oxidation of Ni(II) to Ni(IV) with Aryl Electrophiles Enables Ni-Mediated Aryl-CF3 Coupling. J. Am. Chem. Soc. 2015, 137, 8034–8037.
- 87 Bour, J. R.; Camasso, N. M.; Meucci, E. A.; Kampf, J. W.; Canty, A. J.; Sanford, M. S. Carbon-Carbon Bond-Forming Reductive Elimination from Isolated Nickel(III) Complexes. J. Am. Chem. Soc. 2016, 138, 16105–16111.
- 88 Hu, W.-Q.; Pan, S.; Xu, X.-H.; Vicic, D. A.; Qing, F.-L. Nickel-Mediated Trifluoromethylation of Phenol Derivatives by Aryl C-O Bond Activation. Angew. Chem. Int. Ed. 2020, 59, 16076–16082.
- 89 Xu, C.; Guo, W.-H.; He, X.; Guo, Y.-L.; Zhang, X.-Y.; Zhang, X. Difluoromethylation of (Hetero)Aryl Chlorides with Chlorodifluoromethane Catalyzed by Nickel. Nat. Commun. 2018, 9, 1170.
- 90 Gao, X.; He, X.; Zhang, X. Nickel-Catalyzed Difluoromethylation of (Hetero)aryl Bromides with BrCF2H. Chin. J. Org. Chem. 2019, 39, 215–222.
- 91 Bacauanu, V.; Cardinal, S.; Yamauchi, M.; Kondo, M.; Fernández, D. F.; Remy, R.; MacMillan, D. W. C. Metallaphotoredox Difluoromethylation of Aryl Bromides. Angew. Chem. Int. Ed. 2018, 57, 12543–12548.
- 92 Chi, B. K.; Gavin, S. J.; Ahern, B. N.; Peperni, N.; Monfette, S.; Weix, D. J. Sulfone Electrophiles in Cross-Electrophile Coupling: Nickel-Catalyzed Difluoromethylation of Aryl Bromides. ACS Catal. 2024, 14, 11087–11100.
- 93 He, X.; Gao, X.; Zhang, X. Nickel-Catalyzed Difluoroalkylation of (Hetero)aryl Bromides with Unactivated 1-Bromo-1,1-difluoroalkanes. Chin. J. Chem. 2018, 36, 1059–1062.
- 94 Liu, J.; Zhang, J.; Li, X.; Wu, C.; Liu, H.; Liu, H.; Sun, F.; Li, Y.; Liu, Y.; Li, X. Nickel-Catalyzed 1,1-Difluoroethylation of (Hetero)aryl Halides with 1,1-Difluoroethyl Chloride (CH3CF2Cl). Asian J. Org. Chem. 2020, 9, 391–394.
- 95 Zhang, D.; Gao, X.; Min, Q.-Q.; Gu, Y.; Berthon, G.; Zhang, X. Coupling of Heteroaryl Halides with Chlorodifluoroacetamides and Chlorodifluoroacetate by Nickel Catalysis. Chem. Eur. J. 2022, 28, e202200642.
- 96 Sheng, J.; Ni, H.-Q.; Zhang, H.-R.; Zhang, K.-F.; Wang, Y.-N.; Wang, X.-S. Nickel-Catalyzed Reductive Cross-Coupling of Aryl Halides with Monofluoroalkyl Halides for Late-Stage Monofluoroalkylation. Angew. Chem. Int. Ed. 2018, 57, 7634–7639.
- 97 Yin, H.; Sheng, J.; Zhang, K.-F.; Zhang, Z.-Q.; Bian, K.-J.; Wang, X.-S. Nickel-Catalyzed Monofluoromethylation of (Hetero)Aryl Bromides via Reductive Cross-Coupling. Chem. Commun. 2019, 55, 7635–7638.
- 98 Diao, Z.; Feng, Y.; Zhang, J.; Wang, X.; Li, H.; Ding, C.; Zhou, Z.; Li, X. Nickel-Catalyzed Reductive Cross-Coupling of (Hetero)aryl Halides with 2-Chloro-1,1-difluoroethane: Facile Access to 2,2-Difluoroethylated Aromatics. Asian J. Org. Chem. 2022, 11, e202200169.
- 99 Cui, R.; Sheng, J.; Wu, B.-B.; Hu, D.-D.; Zheng, H.-Q.; Wang, X.-S. Nickel-Catalyzed Reductive Monofluoroakylation of Alkyl Tosylate with Bromofluoromethane to Primary Alkyl Fluoride. Chem. Commun. 2021, 57, 9084–9087.
- 100 Sheng, J.; Ni, H.-Q.; Ni, S.-X.; He, Y.; Cui, R.; Liao, G.-X.; Bian, K.-J.; Wu, B.-B.; Wang, X.-S. Diversity-Oriented Synthesis of Aliphatic Fluorides via Reductive C(sp3)-C(sp3) Cross-Coupling Fluoroalkylation. Angew. Chem. Int. Ed. 2021, 60, 15020–15027.
- 101 Li, H.; Sheng, J.; Wu, B. B.; Li, Y.; Wang, X. S. Nickel-Catalyzed Cross-Coupling of Ethyl Chlorofluoroacetate with Aryl Bromides. Chem. Asian J. 2021, 16, 1741–1744.
- 102 Wang, R.; Xu, J.; Li, J.-X.; Wu, B.-B.; Jin, R.-X.; Bi, Y.-X.; Wang, X.-S. Nickel-Catalyzed Reductive Coupling Reaction of Monofluoroalkyl Triflates with Alkyl Carboxylic Acids toward the Synthesis of α-Alkyl-α-fluoro-alkylketones. Chin. Chem. Lett. 2023, 34, 108490.
- 103 Li, X.; Feng, Z.; Jiang, Z.-X.; Zhang, X. Nickel-Catalyzed Reductive Cross-Coupling of (Hetero)Aryl Iodides with Fluorinated Secondary Alkyl Bromides. Org. Lett. 2015, 17, 5570–5573.
- 104 Min, Y.; Sheng, J.; Yu, J.-L.; Ni, S.-X.; Ma, G.; Gong, H.; Wang, X.-S. Diverse Synthesis of Chiral Trifluoromethylated Alkanes via Nickel- Catalyzed Asymmetric Reductive Cross-Coupling Fluoroalkylation. Angew. Chem. Int. Ed. 2021, 60, 9947–9952.
- 105 Yang, Y.; Luo, G.; Li, Y.; Tong, X.; He, M.; Zeng, H.; Jiang, Y.; Liu, Y.; Zheng, Y. Nickel-Catalyzed Reductive Coupling for Transforming Unactivated Aryl Electrophiles into β-Fluoroethylarenes. Chem. Asian J. 2020, 15, 156–162.
- 106 Li, H.; Sheng, J.; Liao, G.-X.; Wu, B.-B.; Ni, H.-Q.; Li, Y.; Wang, X.-S. Nickel-Catalyzed Direct Trifluoroethylation of Aryl Iodides with 1,1,1-Trifluoro-2-Iodoethane via Reductive Coupling. Adv. Synth. Catal. 2020, 362, 5363–5367.
- 107 Li, X.; Gao, X.; He, C.-Y.; Zhang, X. Using Chlorotrifluoroethane for Trifluoroethylation of (Hetero)aryl Bromides and Chlorides via Nickel Catalysis. Org. Lett. 2021, 23, 1400–1405.
- 108 Jin, R.-X.; Wu, B.-B.; Bian, K.-J.; Yu, J.-L.; Dai, J.-C.; Zuo, Y.-W.; Zhang, Y.-F.; Wang, X.-S. Asymmetric Construction of Allylicstereogenic Carbon Center featuring A Trifluoromethyl Group via Enantioselective Reductive Fluoroalkylation. Nat. Commun. 2022, 13, 7035.
- 109 Wu, B. B.; Xu, J.; Bian, K. J.; Gao, Q.; Wang, X.-S. Enantioselective Synthesis of Secondary β-Trifluoromethyl Alcohols via Catalytic Asymmetric Reductive Trifluoroalkylation and Diastereoselective Reduction. J. Am. Chem. Soc. 2022, 144, 6543–6550.
- 110 Zhao, X.; Tu, H.-Y.; Guo, L.; Zhu, S.; Qing, F.-L.; Chu, L. Intermolecular Selective Carboacylation of Alkenes via Nickel-Catalyzed Reductive Radical Relay. Nat. Commun. 2018, 9, 3488.
- 111 Gu, J.-W.; Min, Q.-Q.; Yu, L.-C.; Zhang, X. Tandem Difluoroalkylation- Arylation of Enamides Catalyzed by Nickel. Angew. Chem. Int. Ed. 2016, 55, 12270–12274.
- 112 Tu, H.-Y.; Wang, F.; Huo, L.; Li, Y.; Zhu, S.; Zhao, X.; Li, H.; Qing, F.-L.; Chu, L. Enantioselective Three-Component Fluoroalkylarylation of Unactivated Olefins through Nickel-Catalyzed Cross-Electrophile Coupling. J. Am. Chem. Soc. 2020, 142, 9604–9611.
- 113
Zhang, X.; Zhao, Q.-W.; Yang, Z.-F.; Fu, X.-P. Access to α,α-Difluoro- γ-amino Acids by Nickel-Catalyzed Reductive Aryldifluoroacetylation of N-Vinylacetamide. Synlett 2020, 32, 1565–1569.
10.1055/s-0040-1706553 Google Scholar
- 114 Wang, F.; Pan, S.; Zhu, S.; Chu, L. Selective Three-Component Reductive Alkylalkenylation of Unbiased Alkenes via Carbonyl-Directed Nickel Catalysis. ACS Catal. 2022, 12, 9779–9789.
- 115 Wang, S.; Guo, B.; Mu, T.; Liu, Z.; He, Y.; Xue, X.-S.; Feng, Z. Iron-Catalyzed Regioselective Reductive Fluoroalkylalkenylation of Unactivated Alkenes. ACS Catal. 2024, 14, 1575–1583.
- 116 Rao, C.; Zhang, T.; Liu, H.; Huang, H. Double Alkyl–Alkyl Bond Construction across Alkenes Enabled by Nickel Electron-Shuttle Catalysis. Nat. Catal. 2023, 6, 847–857.
- 117 Li, Y.-Z.; Rao, N.; An, L.; Wan, X.-L.; Zhang, Y.; Zhang, X. Enantioselective Nickel-Catalyzed Dicarbofunctionalization of 3,3,3-Trifluoropropene. Nat. Commun. 2022, 13, 5539.
- 118 Chen, K.; Liu, Q.; Wan, J.; Zhu, C.; Feng, C. Ni-Catalyzed Reductive Dibenzylation of Trifluoromethylalkenes for CF3-Containing All-Carbon Quaternary Center Construction. Org. Lett. 2023, 25, 5995–6000.
- 119 Domański, S.; Gatlik, B.; Chaładaj, W. Pd-Catalyzed Boroperfluoroalkylation of Alkynes Opens a Route to One-Pot Reductive Carboperfluoroalkylation of Alkynes with Perfluoroalkyl and Aryl Iodides. Org. Lett. 2019, 21, 5021–5025.
- 120 Dai, Y.; Wang, F.; Zhu, S.; Chu, L. Selective Ni-Catalyzed Cross-Electrophile Coupling of Alkynes, Fluoroalkyl Halides, and Vinyl Halides. Chin. Chem. Lett. 2022, 33, 4074–4078.
- 121 Zhang, Z.-Z.; Lei, J.-J.; Zhang, X.-H.; Zhang, X.-G.; Tu, H.-Y. Ni-Catalyzed Reductive Fluoroalkylacylation of Alkynes for the Steroselective Synthesis of Fluoroalkylated Enones. Org. Lett. 2022, 24, 6192–6196.
- 122 Li, H.; Wang, F.; Zhu, S.; Chu, L. Selective Fluoromethyl Couplings of Alkynes via Nickel Catalysis. Angew. Chem. Int. Ed. 2022, 61, e202116725.




