Boosting Photogenerated Electrons Transfer from FeVO4 to Peroxymonosulfate via Cu Doping for Stable Degradation of Organic Contaminants
Zhou Zhong
Fujian Provincial Key Laboratory of Soil Environmental Health and Regulation, College of Resources and Environment, Fujian Agriculture and Forestry University, Fuzhou, Fujian, 350002 China
State Key Laboratory of Photocatalysis on Energy and Environment, Fuzhou University, Fuzhou, Fujian, 350116 China
Search for more papers by this authorXiang-Ji Liu
Fujian Provincial Key Laboratory of Soil Environmental Health and Regulation, College of Resources and Environment, Fujian Agriculture and Forestry University, Fuzhou, Fujian, 350002 China
Search for more papers by this authorLi Ma
Fujian Provincial Key Laboratory of Soil Environmental Health and Regulation, College of Resources and Environment, Fujian Agriculture and Forestry University, Fuzhou, Fujian, 350002 China
Search for more papers by this authorZi-Jian Zhan
Fujian Provincial Key Laboratory of Soil Environmental Health and Regulation, College of Resources and Environment, Fujian Agriculture and Forestry University, Fuzhou, Fujian, 350002 China
Search for more papers by this authorYu-Xin Yuan
Fujian Provincial Key Laboratory of Soil Environmental Health and Regulation, College of Resources and Environment, Fujian Agriculture and Forestry University, Fuzhou, Fujian, 350002 China
Search for more papers by this authorHeng-Jian Zhang
Fujian Provincial Key Laboratory of Soil Environmental Health and Regulation, College of Resources and Environment, Fujian Agriculture and Forestry University, Fuzhou, Fujian, 350002 China
Search for more papers by this authorCorresponding Author
Feng-Ying Cai
Fujian Provincial Key Laboratory of Soil Environmental Health and Regulation, College of Resources and Environment, Fujian Agriculture and Forestry University, Fuzhou, Fujian, 350002 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorYi-Dong Hou
State Key Laboratory of Photocatalysis on Energy and Environment, Fuzhou University, Fuzhou, Fujian, 350116 China
Search for more papers by this authorCorresponding Author
Jian Lü
Fujian Provincial Key Laboratory of Soil Environmental Health and Regulation, College of Resources and Environment, Fujian Agriculture and Forestry University, Fuzhou, Fujian, 350002 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Rong Cao
State Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou, Fujian, 350002 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorZhou Zhong
Fujian Provincial Key Laboratory of Soil Environmental Health and Regulation, College of Resources and Environment, Fujian Agriculture and Forestry University, Fuzhou, Fujian, 350002 China
State Key Laboratory of Photocatalysis on Energy and Environment, Fuzhou University, Fuzhou, Fujian, 350116 China
Search for more papers by this authorXiang-Ji Liu
Fujian Provincial Key Laboratory of Soil Environmental Health and Regulation, College of Resources and Environment, Fujian Agriculture and Forestry University, Fuzhou, Fujian, 350002 China
Search for more papers by this authorLi Ma
Fujian Provincial Key Laboratory of Soil Environmental Health and Regulation, College of Resources and Environment, Fujian Agriculture and Forestry University, Fuzhou, Fujian, 350002 China
Search for more papers by this authorZi-Jian Zhan
Fujian Provincial Key Laboratory of Soil Environmental Health and Regulation, College of Resources and Environment, Fujian Agriculture and Forestry University, Fuzhou, Fujian, 350002 China
Search for more papers by this authorYu-Xin Yuan
Fujian Provincial Key Laboratory of Soil Environmental Health and Regulation, College of Resources and Environment, Fujian Agriculture and Forestry University, Fuzhou, Fujian, 350002 China
Search for more papers by this authorHeng-Jian Zhang
Fujian Provincial Key Laboratory of Soil Environmental Health and Regulation, College of Resources and Environment, Fujian Agriculture and Forestry University, Fuzhou, Fujian, 350002 China
Search for more papers by this authorCorresponding Author
Feng-Ying Cai
Fujian Provincial Key Laboratory of Soil Environmental Health and Regulation, College of Resources and Environment, Fujian Agriculture and Forestry University, Fuzhou, Fujian, 350002 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorYi-Dong Hou
State Key Laboratory of Photocatalysis on Energy and Environment, Fuzhou University, Fuzhou, Fujian, 350116 China
Search for more papers by this authorCorresponding Author
Jian Lü
Fujian Provincial Key Laboratory of Soil Environmental Health and Regulation, College of Resources and Environment, Fujian Agriculture and Forestry University, Fuzhou, Fujian, 350002 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Rong Cao
State Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou, Fujian, 350002 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorComprehensive Summary
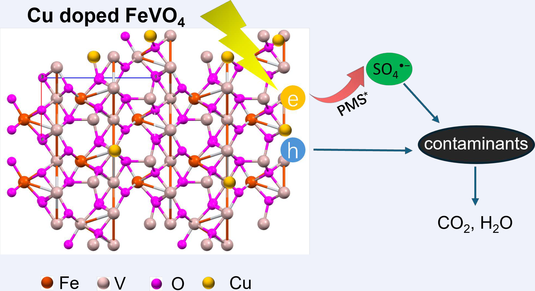
Electron transfer is an important way to activate persulfate. Currently, the electrons for persulfate activation mainly originate from organic contaminants or the catalyst itself, which can lead to selective activation of persulfate or oxidation of the catalyst, respectively, and thus become a bottleneck restricting its application. In this work, Cu−doped FeVO4 (Cu−FVO) was prepared, and the results showed that Cu doping can significantly improve the photocatalytic activity and stability of FVO for peroxymonosulfate (PMS) activation. The optimized Cu−FVO/PMS/light system exhibited a high BPA degradation rate that is 4.3 times higher than that of the FVO/PMS/light. This system manifested a broad applicability to various organic contaminants even with complex matrix. Photoelectrochemical analysis and DFT theoretical calculations revealed that Cu doping boosted the photogenerated charge separation and the adsorption of PMS on FVO. Furthermore, Cu doping led to the establishment of an electron transfer channel from Cu−FVO to PMS, through which photogenerated electrons achieved an efficient PMS activation. Meanwhile, holes were consumed by organic contaminants to avoid the oxidation of catalyst. These collectively enhanced the photocatalytic activity and stability of Cu−FVO, which also maintained high catalytic activity even after 20 cycling degradation reactions.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202400669-sup-0001-supinfo.pdfPDF document, 1.1 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Cao, Z.; Chen, Q.; Zhu, C.; Chen, X.; Wang, N.; Zou, W.; Zhang, X.; Zhu, G.; Li, J.; Mai, B.; Luo, X. Halogenated organic pollutant residuals in human bared and clothing−covered skin areas: Source differentiation and comprehensive health risk assessment. Environ. Sci. Technol. 2019, 53, 14700–14708.
- 2 Lapworth, D. J.; Baran, N.; Stuart, M. E.; Ward, R. S. Emerging organic contaminants in groundwater: A review of sources, fate and occurrence. Environ. Pollut. 2012, 163, 287–303.
- 3 Hu, X.; Zhu, M. Were persulfate−based advanced oxidation processes really understood? Basic concepts, cognitive Biases, and experimental details. Environ. Sci. Technol. 2024, 58, 10415–10444.
- 4 Yang, J.; Zhu, M.; Dionysiou, D. D. What is the role of light in persulfate−based advanced oxidation for water treatment? Water Res. 2021, 189, 116627.
- 5 Lee, J.; Von Gunten, U.; Kim, J. H. Persulfate−based advanced oxidation: critical assessment of opportunities and roadblocks. Environ. Sci. Technol. 2020, 54, 3064–3081.
- 6 Wang, W.; Liu, Y.; Yue, Y.; Wang, H.; Cheng, G.; Gao, C.; Chen, C.; Ai, Y.; Chen, Z.; Wang, X. The confined interlayer growth of ultrathin two−dimensional Fe3O4 nanosheets with enriched oxygen vacancies for peroxymonosulfate activation. ACS Catal. 2021, 11, 11256–11265.
- 7 Bu, Y.; Li, H.; Yu, W.; Pan, Y.; Li, L.; Wang, Y.; Pu, L.; Ding, J.; Gao, G.; Pan, B. Peroxydisulfate activation and singlet oxygen generation by oxygen vacancy for degradation of contaminants. Environ. Sci. Technol. 2021, 55, 2110–2120.
- 8 Wang, J.; Wang, S. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chem. Eng. J. 2018, 334, 1502–1517.
- 9 Chen, Y.; Kang, J.; Li, Z.; Zhu, M.; Yin, R. Activation of peroxymonosulfate by Co2P via interfacial radical pathway for the degradation and mineralization of carbamazepine. Surf. Interfaces 2023, 40, 103045.
- 10 Zhang, J.; Liu, W.; Liu, B.; Duan, X.; Ao, Z.; Zhu, M. Is single−atom catalyzed peroxymonosulfate activation better? Coupling with metal oxide may be better. Chin. J. Catal. 2024, 59, 137–148.
- 11 Ren, W.; Cheng, C.; Shao, P.; Luo, X.; Zhang, H.; Wang, S.; Duan, X. Origins of electron−transfer regime in persulfate−based nonradical oxidation processes. Environ. Sci. Technol. 2022, 56, 78–97.
- 12 Shao, P.; Yu, S.; Duan, X.; Yang, L.; Shi, H.; Ding, L.; Tian, J.; Yang, L.; Luo, X.; Wang, S. Potential difference driving electrontransfer via defective carbon nanotubes toward selective oxidation of organic micropollutants. Environ. Sci. Technol. 2020, 54, 8464–8472.
- 13 Wei, H.; Loeb, S. K.; Halas, N. J.; Kim, J. H. Plasmon−enabled degradation of organic micropollutants in water by visible−light illumination of Janus gold nanorods. Proc. Natl. Acad. Sci. U. S. A. 2020, 117, 15473–15481.
- 14 Ren, W.; Xiong, L.; Yuan, X.; Yu, Z.; Zhang, H.; Duan, X.; Wang, S. Activation of peroxydisulfate on carbon nanotubes: electron−transfer mechanism. Environ. Sci. Technol. 2019, 53, 14595–14603.
- 15 Liu, X.; Qiao, X.; Yang, R.; Wei, D.; Qu, X.; Cao, H.; Li, Y.; Zhong, Z.; Lu, J. Mechanism insights into photo−assisted peroxymonosulfate activation on oxygen vacancy−enriched nolanites via an electron transfer regime. J. Colloid Interface Sci. 2023, 652, 912–922.
- 16 Li, H.; Shan, C.; Li, W.; Pan, B. Peroxymonosulfate activation by iron(III)−tetraamidomacrocyclic ligand for degradation of organic pollutants via high−valent iron−oxo complex. Water Res. 2018, 147, 233–241.
- 17 Wang, Z.; Jiang, J.; Pang, S.; Zhou, Y.; Guan, C.; Gao, Y.; Li, J.; Yang, Y.; Qiu, W.; Jiang, C. Is sulfate radical really generated from peroxydisulfate activated by iron(II) for environmental decontamination? Environ. Sci. Technol. 2018, 52, 11276–11284.
- 18 Ike, I. A.; Linden, K. G.; Orbell, J. D.; Duke, M. Critical review of the science and sustainability of persulphate advanced oxidation processes. Chem. Eng. J. 2018, 338, 651–669.
- 19 Li, N.; Lu, W.; Pei, K.; Yao, Y.; Chen, W. Formation of high−valent cobalt−oxo phthalocyanine species in a cellulose matrix for eliminating organic pollutants. Appl. Catal. B 2015, 163, 105–112.
- 20 Xu, L.; Qi, L.; Sun, Y.; Gong, H.; Chen, Y.; Pei, C.; Gan, L. Mechanistic studies on peroxymonosulfate activation by g-C3N4 under visible light for enhanced oxidation of light−inert dimethyl phthalate. Chin. J. Catal. 2020, 41, 322–332.
- 21 Hasija, V.; Nguyen, V. −H.; Kumar, A.; Raizada, P.; Krishnan, V.; Khan, A. A. P.; Singh, P.; Lichtfouse, E.; Wang, C.; Thi Huong, P. Advanced activation of persulfate by polymeric g-C3N4 based photocatalysts for environmental remediation: A review. J. Hazard. Mater. 2021, 413, 125324.
- 22 Chen, G.; Yu, Y.; Liang, L.; Duan, X.; Li, R.; Lu, X.; Yan, B.; Li, N.; Wang, S. Remediation of antibiotic wastewater by coupled photocatalytic and persulfate oxidation system: A critical review. J. Hazard. Mater. 2021, 408, 124461.
- 23 Xing, Y.; Jiang, X.; Han, L.; Jin, X.; Ni, G.; Peng, Y.; Yong, X.; Wang, X. Efficient degradation of tetracycline over vacancy−modified Cu-doped Bi2O2S via peroxymonosulfate activation and photocatalysis. J. Clean. Prod. 2023, 400, 136631.
- 24 Shi, H.; He, Y.; Li, Y.; Luo, P. Unraveling the synergy mechanism between photocatalysis and peroxymonosulfate activation on a Co/Fe bimetal-doped carbon nitride. ACS Catal. 2023, 13, 8973–8986.
- 25 Sayed, M.; Ren, B.; Ali, A. M.; Al-Anazi, A.; Nadagouda, M. N.; Ismail, A. A.; Dionysiou, D. D. Solar light induced photocatalytic activation of peroxymonosulfate by ultra-thin Ti3+ self-doped Fe2O3/TiO2 nanoflakes for the degradation of naphthalene. Appl. Catal. B 2022, 315, 121532.
- 26 Wang, G.; Huo, T.; Deng, Q.; Yu, F.; Xia, Y.; Li, H.; Hou, W. Surface- layer bromine doping enhanced generation of surface oxygen vacancies in bismuth molybdate for efficient photocatalytic nitrogen fixation. Appl. Catal. B 2022, 310, 121319.
- 27 Huang, J.; Kang, Y.; Liu, J.; Yao, T.; Qiu, J.; Du, P.; Huang, B.; Hu, W.; Liang, Y.; Xie, T.; Chen, C.; Yin, L.-C.; Wang, L.; Cheng, H.-M.; Liu, G. Gradient tungsten-doped Bi3TiNbO9 ferroelectric photocatalysts with additional built-in electric field for efficient overall water splitting. Nat. Commun. 2023, 14, 7948–7948.
- 28
Li, R.; Takata, T.; Zhang, B.; Feng, C.; Wu, Q.; Cui, C.; Zhang, Z.; Domen, K.; Li, Y. Criteria for efficient photocatalytic water splitting revealed by studying carrier dynamics in a model Al-doped SrTiO3 photocatalyst. Angew. Chem. Int. Ed. 2023, 62, 202312537.
10.1002/anie.202313537 Google Scholar
- 29 Murthy, D. H. K.; Nandal, V.; Furube, A.; Seki, K.; Katoh, R.; Lyu, H.; Hisatomi, T.; Domen, K.; Matsuzaki, H. Origin of enhanced overall water splitting efficiency in aluminum-doped SrTiO3 photocatalyst. Adv. Energy Mater. 2023, 13, 2302064.
- 30 Yang, Z.; Yang, X.; An, G.; Wang, D. Regulating spin state of Fe active sites by the P-doping strategy for enhancing peroxymonosulfate activation. Appl. Catal. B 2023, 330, 122618.
- 31
Zhan, H.; Zhou, R.; Wang, P.; Zhou, Q. Selective hydroxyl generation for efficient pollutant degradation by electronic structure modulation at Fe sites. Proc. Natl. Acad. Sci. U. S. A. 2023, 120, 2305378120.
10.1073/pnas.2305378120 Google Scholar
- 32
Yang, P.; Long, Y.; Huang, W.; Liu, D. Single-atom copper embedded in two-dimensional MXene toward peroxymonosulfate activation to generate singlet oxygen with nearly 100% selectivity for enhanced Fenton-like reactions. Appl. Catal. B 2023, 324, 122445.
10.1016/j.apcatb.2022.122245 Google Scholar
- 33 Long, X.; Gao, L.; Li, F.; Hu, Y.; Wei, S.; Wang, C.; Wang, T.; Jin, J.; Ma, J. Bamboo shoots shaped FeVO4 passivated ZnO nanorods photoanode for improved charge separation/transfer process towards efficient solar water splitting. Appl. Catal. B 2019, 257, 117813.
- 34 Zhang, J.; Zhao, W.; Li, Z.; Lu, G.; Zhu, M. Visible−light−assisted peroxymonosulfate activation over Fe(II)/V(IV) self-doped FeVO4 nanobelts with enhanced sulfamethoxazole degradation: Performance and mechanism. Chem. Eng. J. 2021, 403, 126384.
- 35 Chachvalvutikul, A.; Jakmunee, J.; Thongtem, S.; Kittiwachana, S.; Kaowphong, S. Novel FeVO4/Bi7O9I3 nanocomposite with enhanced photocatalytic dye degradation and photoelectrochemical properties. Appl. Surf. Sci. 2019, 475, 175–184.
- 36 Yang, R.; Zhu, Z.; Hu, C.; Zhong, S.; Zhang, L.; Liu, B.; Wang, W. One−step preparation (3D/2D/2D) BiVO4/FeVO4@rGO heterojunction composite photocatalyst for the removal of tetracycline and hexavalent chromium ions in water. Chem. Eng. J. 2020, 390, 124522.
- 37 Wang, J.-T.; Cai, Y.-L.; Liu, X.-J.; Zhang, X.-D.; Cai, F.-Y.; Cao, H.-L.; Zhong, Z.; Li, Y.-F.; Lü, J. Unveiling the visible-light-driven photodegradation pathway and products toxicity of tetracycline in the system of Pt/BiVO4 nanosheets. J. Hazard. Mater. 2022, 424, 127596.
- 38 Tawfik, A.; Alalm, M. G.; Awad, H. M.; Islam, M.; Qyyum, M. A.; Al-Muhtaseb, A. a. H.; Osman, A. I.; Lee, M. Solar photo-oxidation of recalcitrant industrial wastewater: a review. Environ. Chem. Lett. 2022, 20, 1839–1862.
- 39 Chen, H.; Xu, Y.; Zhu, K.; Zhang, H. Understanding oxygen-deficient La2CuO4−δ perovskite activated peroxymonosulfate for bisphenol A degradation: The role of localized electron within oxygen vacancy. Appl. Catal. B 2021, 284, 119732.
- 40 Yang, J.; Xie, T.; Mei, Y.; Chen, J.; Sun, H.; Feng, S.; Zhang, Y.; Zhao, Y.; Wang, J.; Li, X.; He, J.; Chen, H. High-efficiency V-mediated Bi2MoO6 photocatalyst for PMS activation: Modulation of energy band structure and enhancement of surface reaction. Appl. Catal. B 2023, 339, 123149.
- 41 Gao, L.; Guo, Y.; Zhan, J.; Yu, G.; Wang, Y. Assessment of the validity of the quenching method for evaluating the role of reactive species in pollutant abatement during the persulfate-based process. Water Res. 2022, 221, 118730.
- 42 Zhong, Z.; Liu, J.; Xu, X.; Cao, A.; Tao, Z.; You, W.; Kang, L. Synthesis of Z-scheme cobalt porphyrin/nitrogen-doped graphene quantum dot heterojunctions for efficient molecule-based photocatalytic oxygen evolution. J. Mater. Chem. A 2021, 9, 2404–2413.
- 43 Zhao, Y.; Yu, L.; Song, C.; Chen, Z.; Meng, F.; Song, M. Selective degradation of electron-rich organic pollutants induced by CuO@Biochar: The key role of outer−sphere interaction and singlet oxygen. Environ. Sci. Technol. 2022, 10710–10720.
- 44 Guo, Y.; Long, J.; Huang, J.; Yu, G.; Wang, Y. Can the commonly used quenching method really evaluate the role of reactive oxygen species in pollutant abatement during catalytic ozonation? Water Res. 2022, 215, 118275.
- 45 Hu, J.; Zeng, X.; Wang, G.; Qian, B.; Liu, Y.; Hu, X.; He, B.; Zhang, L.; Zhang, X. Modulating mesoporous Co3O4 hollow nanospheres with oxygen vacancies for highly efficient peroxymonosulfate activation. Chem. Eng. J. 2020, 400, 125869.
- 46 Xie, X.; Cao, J.; Xiang, Y.; Xie, R.; Suo, Z.; Ao, Z.; Yang, X.; Huang, H. Accelerated iron cycle inducing molecular oxygen activation for deep oxidation of aromatic VOCs in MoS2 co-catalytic Fe3+/PMS system. Appl. Catal. B 2022, 309, 121235.
- 47 Criquet, J.; Leitner, N. K. V. Degradation of acetic acid with sulfate radical generated by persulfate ions photolysis. Chemosphere 2009, 77, 194–200.
- 48 Wu, S.; Chen, Z.; Yue, W.; Mine, S.; Toyao, T.; Matsuoka, M.; Xi, X.; Wang, L.; Zhang, J. Single-atom high-valent Fe(IV) for promoted photocatalytic nitrogen hydrogenation on porous TiO2-SiO2. ACS Catal. 2021, 11, 4362–4371.
- 49 Wang, S.; He, T.; Chen, P.; Du, A.; Ostrikov, K. K.; Huang, W.; Wang, L. In situ formation of oxygen vacancies achieving near-complete charge separation in planar BiVO4 photoanodes. Adv. Mater. 2020, 32, 2001385.
- 50 Zhang, Z.; Wang, W.; Gao, E.; Shang, M.; Xu, J. Enhanced photocatalytic activity of Bi2WO6 with oxygen vacancies by zirconium doping. J. Hazard. Mater. 2011, 196, 255–62.
- 51 Yang, Z.; Shi, Y.; Li, H.; Mao, C.; Wang, X.; Liu, X.; Liu, X.; Zhang, L. Oxygen and chlorine dual vacancies enable photocatalytic O2 dissociation into monatomic reactive oxygen on BiOCl for refractory aromatic pollutant removal. Environ. Sci. Technol. 2022, 56, 3587–3595.
- 52
Guo, Z. Y.; Sun, R.; Huang, Z.; Han, X.; Wang, H.; Chen, C.; Liu, Y. Q.; Zheng, X.; Zhang, W.; Hong, X.; Li, W. W. Crystallinity engineering for overcoming the activity-stability tradeoff of spinel oxide in Fenton- like catalysis. Proc. Natl. Acad. Sci. U. S. A. 2023, 120, 2220608120.
10.1073/pnas.2220608120 Google Scholar
- 53 Zhang, P.; Yang, Y.; Duan, X.; Liu, Y.; Wang, S. Density functional theory calculations for insight into the heterocatalyst reactivity and mechanism in persulfate−based advanced oxidation reactions. ACS Catal. 2021, 11, 11129–11159.
- 54 Wu, X. L.; Liu, S.; Li, Y.; Yan, M.; Lin, H.; Chen, J.; Liu, S.; Wang, S.; Duan, X. Directional and ultrafast charge transfer in oxygen-vacancy- rich ZnO@single−Atom cobalt core−shell junction for photo-Fenton- like reaction. Angew. Chem. Int. Ed. 2023, 62, 202305639.




