Catalytic Cycloaddition Reactions of Ynol and Thioynol Ethers
Corresponding Author
Ming-Yu Teng
College of Chemistry and Chemical Engineering, Yunnan Normal University, Kunming, Yunnan, 650500 China
E-mail: [email protected]; [email protected]Search for more papers by this authorYin Xu
Key Laboratory of Chemical Biology of Fujian Province, State Key Laboratory of Physical Chemistry of Solid Surfaces, and College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, Fujian, 361005 China
Search for more papers by this authorXin-Qi Zhu
College of Chemistry and Chemical Engineering, Yunnan Normal University, Kunming, Yunnan, 650500 China
Search for more papers by this authorBo Zhou
Key Laboratory of Chemical Biology of Fujian Province, State Key Laboratory of Physical Chemistry of Solid Surfaces, and College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, Fujian, 361005 China
Search for more papers by this authorCorresponding Author
Long-Wu Ye
College of Chemistry and Chemical Engineering, Yunnan Normal University, Kunming, Yunnan, 650500 China
Key Laboratory of Chemical Biology of Fujian Province, State Key Laboratory of Physical Chemistry of Solid Surfaces, and College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, Fujian, 361005 China
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, Shanghai, 200032 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Ming-Yu Teng
College of Chemistry and Chemical Engineering, Yunnan Normal University, Kunming, Yunnan, 650500 China
E-mail: [email protected]; [email protected]Search for more papers by this authorYin Xu
Key Laboratory of Chemical Biology of Fujian Province, State Key Laboratory of Physical Chemistry of Solid Surfaces, and College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, Fujian, 361005 China
Search for more papers by this authorXin-Qi Zhu
College of Chemistry and Chemical Engineering, Yunnan Normal University, Kunming, Yunnan, 650500 China
Search for more papers by this authorBo Zhou
Key Laboratory of Chemical Biology of Fujian Province, State Key Laboratory of Physical Chemistry of Solid Surfaces, and College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, Fujian, 361005 China
Search for more papers by this authorCorresponding Author
Long-Wu Ye
College of Chemistry and Chemical Engineering, Yunnan Normal University, Kunming, Yunnan, 650500 China
Key Laboratory of Chemical Biology of Fujian Province, State Key Laboratory of Physical Chemistry of Solid Surfaces, and College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, Fujian, 361005 China
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, Shanghai, 200032 China
E-mail: [email protected]; [email protected]Search for more papers by this authorAbstract
Comprehensive Summary
Electron-rich alkynes, such as ynol and thioynol ethers, have proven to be versatile and appealing partners in catalytic cycloaddition reactions, and thus have raised considerable attentions owing to the practical application in the modular assembly of valuable carbo- and heterocycles. The past decades have witnessed inspiring advances in this emerging field, and an increasing number of related discoveries have been exploited. Divided into two main sections on the basis of substrate type, in each section this comprehensive review will initially summarize their synthetic preparations and subsequently examine their reactivity in every sort of catalytic cycloaddition with emphasis on the methodology development, aimed at providing an access to this burgeoning area and encouraging further innovations in the near future.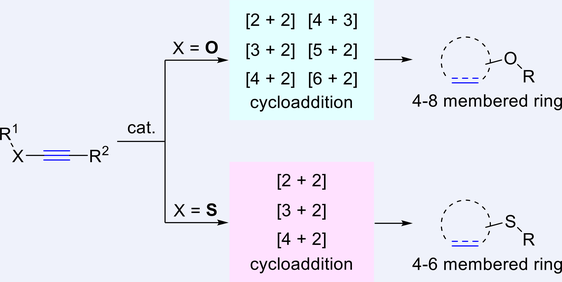
Key Scientists
For the cycloaddition of ynol ethers, in 2004, Kozmin et al. firstly developed a silver-catalyzed [2 + 2] cycloaddition of siloxy alkynes with electron-poor olefins. In 2012, Hiyama et al. realized a palladium-catalyzed formal [4 + 2] annulation of alkynyl aryl ethers with internal alkynes. In the same year, Sun et al. discovered an efficient [6 + 2] cyclization between siloxy alkynes and 2-(oxetan-3-yl)benzaldehydes by applying HNTf2 as catalyst. In 2017, Wender et al. first utilized vinylcyclopropanes (VCPs) as coupling partners in the [5 + 2] annulation of ynol ethers. In 2018 and 2020, Ye et al. reported zinc-catalyzed formal [3 + 2] and [4 + 3] cycloaddition, respectively. For the cycloaddition of thioynol ethers, in 2004, Hilt et al. realized a [4 + 2] cycloaddition by employing the alkynyl sulfides and acyclic 1,3-dienes. In 2006, a ruthenium-catalyzed [2 + 2] cycloaddition of thioynol ethers with bicyclic alkenes was accomplished by Tam. In 2014, Sun et al. reported an elegant iridium-catalyzed click reaction of thioalkynes with azides.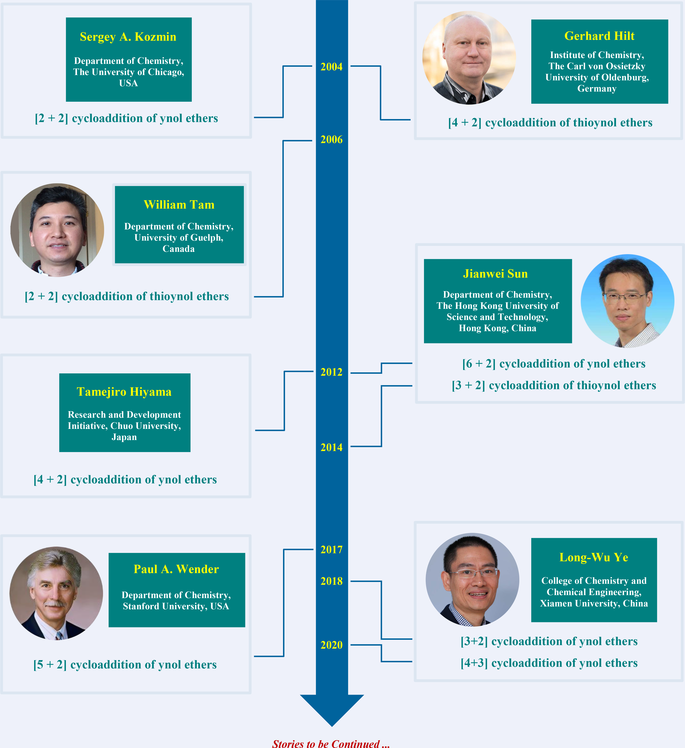
References
- 1For recent selected reviews on ynamide reactivity, see: (a) Hu, L.; Zhao, J. Ynamide Coupling Reagents: Origin and Advances. Acc. Chem. Res. 2024, 57, 855–869; (b) Hu, Y.-C.; Zhao, Y.; Wan, B.; Chen, Q.-A. Reactivity of Ynamides in Catalytic Intermolecular Annulations. Chem. Soc. Rev. 2021, 50, 2582–2625; (c) Lynch, C. C.; Sripada, A.; Wolf, C. Asymmetric Synthesis with Ynamides: Unique Reaction Control, Chemical Diversity and Applications. Chem. Soc. Rev. 2020, 49, 8543–8583; (d) Chen, Y.-B.; Qian, P.-C.; Ye, L.-W. Brønsted Acid-Mediated Reactions of Ynamides. Chem. Soc. Rev. 2020, 49, 8897–8909; (e) Hong, F.-L.; Ye, L.-W. Transition Metal-Catalyzed Tandem Reactions of Ynamides for Divergent N-Heterocycle Synthesis. Acc. Chem. Res. 2020, 53, 2003–2019; (f) Luo, J.; Chen, G.-S.; Chen, S.-J.; Yu, J.-S.; Li, Z.-D.; Liu, Y.-L. Exploiting Remarkable Reactivities of Ynamides: Opportunities in Designing Catalytic Enantioselective Reactions. ACS Catal. 2020, 10, 13978–13992; (g) Zhou, B.; Tan, T.-D.; Zhu, X.-Q.; Shang, M.; Ye, L.-W. Reversal of Regioselectivity in Ynamide Chemistry. ACS Catal. 2019, 9, 6393–6406; (h) Evano, G.; Theunissen, C.; Lecomte, M. Ynamides: Powerful and Versatile Reagents for Chemical Synthesis. Aldrichim. Acta 2015, 48, 59–70; (i) Wang, X.-N.; Yeom, H.-S.; Fang, L.-C.; He, S.; Ma, Z.-X.; Kedrowski, B. L.; Hsung, R. P. Ynamides in Ring Forming Transformations. Acc. Chem. Res. 2014, 47, 560–578; (j) DeKorver, K. A.; Li, H.; Lohse, A. G.; Hayashi, R.; Lu, Z.; Zhang, Y.; Hsung, R. P. Ynamides: A Modern Functional Group for the New Millennium. Chem. Rev. 2010, 110, 5064–5106; (k) Evano, G.; Coste, A.; Jouvin, K. Ynamides: Versatile Tools in Organic Synthesis. Angew. Chem. Int. Ed. 2010, 49, 2840–2859.
- 2 Minehan, T. G. Tandem Bond-Forming Reactions of 1-Alkynyl Ethers. Acc. Chem. Res. 2016, 49, 1168–1181.
- 3 Gray, V. J.; Wilden, J. D. The Chemistry of Ynol and Thioynol Ethers. Org. Biomol. Chem. 2016, 14, 9695–9711.
- 4For recent selected examples on thioynol ethers, see: (a) Sharma, P.; Singh, R. R.; Giri, S. S.; Chen, L.-Y.; Cheng, M.-J.; Liu, R.-S. Gold-Catalyzed Oxidation of Thioalkynes to Form Phenylthio Ketene Derivatives via a Noncarbene Route. Org. Lett. 2019, 21, 5475–5479; (b) Zhang, Y.-Q.; Zhu, X.-Q.; Chen, Y.-B.; Tan, T.-D.; Yang, M.-Y.; Ye, L.-W. Synthesis of Isothiochroman-3-ones via Metal-Free Oxidative Cyclization of Alkynyl Thioethers. Org. Lett. 2018, 20, 7721–7725; (c) Zhu, X.-Q.; Sun, Q.; Zhang, Z.-X.; Zhou, B.; Xie, P.-X.; Shen, W.-B.; Lu, X.; Zhou, J.-M.; Ye, L.-W. Zinc-Catalyzed Reaction of Isoxazoles with Thioynol Ethers Involving an Unprecedented 1,2-Sulfur Migration. Chem. Commun. 2018, 54, 7435–7438.
- 5For recent selected examples on ynol ethers, see: (a) Misawa, S.; Miyairi, A.; Oonishi, Y.; Nolan, S. P.; Sato, Y. Synthesis of γ,δ-Unsaturated Esters and Amides via Au(I)-Catalyzed Reactions of Aryl Ynol Ethers or Ynamides with Allylic Alcohols. Synthesis 2021, 53, 4644–4653; (b) Zhu, H.; Jin, W.; He, J.; Zhang, Y.; Zhu, G. Synthesis of (E)-α,β-Unsaturated Carbonyls via Silver-Catalyzed Tandem Epoxide Rearrangement/Intermolecular Carbonyl-Heteroalkyne Metathesis. Adv. Synth. Catal. 2016, 358, 3730–3735.
- 6For recent example on ynol ethers, see: (a) Minami, Y.; Noguchi, Y.; Hiyama, T. Synthesis of Benzosiloles by Intramolecular anti-Hydroarylation via ortho-C–H Activation of Aryloxyethynyl Silanes. J. Am. Chem. Soc. 2017, 139, 14013–14016. For recent example on thioynol ethers, see: (b) Luz, E. Q.; Silvério, G. L.; Seckler, D.; Lima, D. B.; Santana, F. S.; Barbosa, R. V.; D'Oca, R. M.; Rampon, D. S. One-Pot Synthesis of 3-Halo-2-organochalcogenylbenzo[b]chalcogenophenes from 1-(2,2-Dibromovinyl)-2-organochalcogenylbenzenes. Adv. Synth. Catal. 2021, 363, 2610–2618.
- 7For recent selected examples on ynol ethers, see: (a) Liu, Y.; Han, C.; Shi, H.; Mackenroth, A. V.; Zhang, L.; Rudolph, M. Gold-Catalyzed Regio- and Stereoselective α-Acyloxy-β-Alkynylation of Ynol Ethers. J. Org. Chem. 2023, 88, 2908–2920; (b) Zeng, L.; Sajiki, H.; Cui, S. One-Pot Reaction of Carboxylic Acids, Ynol Ethers, and m-CPBA for Synthesis of α-Carbonyloxy Esters. Org. Lett. 2019, 21, 6423–6426; (c) Zeng, L.; Huang, B.; Shen, Y.; Cui, S. Multicomponent Synthesis of Tetrahydroisoquinolines. Org. Lett. 2018, 20, 3460–3464; (d) Zeng, L.; Lai, Z.; Cui, S. One-Pot Reaction of Carboxylic Acids and Ynol Ethers for The Synthesis of β-Keto Esters. J. Org. Chem. 2018, 83, 14834–14841. For recent selected examples on thioynol ethers, see: (e) Wang, Y.; Li, Y.; Wang, L.; Ding, S.; Song, L.; Zhang, X.; Wu, Y.-D.; Sun, J. Ir-Catalyzed Regioselective Dihydroboration of Thioalkynes toward Gem-Diboryl Thioethers. J. Am. Chem. Soc. 2023, 145, 2305–2314; (f) Ye, X.; Wang, J.; Ding, S.; Hosseyni, S.; Wojtas, L.; Akhmedov, N. G.; Shi, X. Investigations on Gold-Catalyzed Thioalkyne Activation Toward Facile Synthesis of Ketene Dithioacetals. Chem. - Eur. J. 2017, 23, 10506–10510.
- 8
Löffler, A.; Himbert, G. Alkoxyacetylenes from Alkyl 1,2-Dichlorovinyl Ethers. Synthesis 1992, 495–498.
10.1055/s-1992-26145 Google Scholar
- 9 Christopher, A.; Brandes, B.; Kelly, S.; Minehan, T. G. Low Temperature n-Butyllithium Induced Rearrangement of Allyl-1,1-Dichlorovinyl Ethers. Org. Lett. 2006, 8, 451–454.
- 10 Sosa, J. R.; Tudjarian, A. A.; Minehan, T. G. Synthesis of Alkynyl Ethers and Low-Temperature Sigmatropic Rearrangement of Allyl and Benzyl Alkynyl Ethers. Org. Lett. 2008, 10, 5091–5094.
- 11 Jouvin, K.; Bayle, A.; Legrand, F.; Evano, G. Copper-Catalyzed Coupling of 1,1-Dibromo-1-alkenes with Phenols: A General, Modular, and Efficient Synthesis of Ynol Ethers, Bromo Enol Ethers, and Ketene Acetals. Org. Lett. 2012, 14, 1652–1655.
- 12(a) Tanaka, R.; Miller, S. I. Nucleophilic Substitution at an Acetylenic Carbon: 1-Alkoxy-2-phenylacetylenes from 1-Chloro-2-phenylacetylene. Tetrahedron Lett. 1971, 12, 1753–1756;
10.1016/S0040-4039(01)87452-9 Google Scholar(b) Gulia, N.; Pigulski, B.; Charewicz, M.; Szafert, S. A Versatile and Highly Efficient Method for 1-Chlorination of Terminal and Trialkylsilyl-Protected Alkynes. Chem. - Eur. J. 2014, 20, 2746–2749.
- 13(a) Gray, V. J.; Slater, B.; Wilden, J. D. Transition-Metal-Free Synthesis of Aryl-Substituted tert-Butyl Ynol Ethers through Addition/Elimination Substitution at an sp Centre. Chem. - Eur. J. 2012, 18, 15582–15585; (b) Marzo, L.; Parra, A.; Frías, M.; Alemán, J.; Ruano, J. L. G. Synthesis of Alkyl-Ynol-Ethers by “Anti-Michael Addition” of Metal Alkoxides to β-Substituted Alkynylsulfones. Eur. J. Org. Chem. 2013, 2013, 4405–4409; (c) Gray, V. J.; Cuthbertson, J.; Wilden, J. D. Transition-Metal-Free Synthesis of Ynol Ethers and Thioynol Ethers via Displacement at sp Centers: A Revised Mechanistic Pathway. J. Org. Chem. 2014, 79, 5869–5874.
- 14(a) Ficini, J. Studies on Ethers of Ynols. Preparation and Reactions with Compounds Having an Active Hydrogen. Bull. Soc. Chim. Fr. 1954, 1367–1371;
(b) Nieuwenhuis, J.; Arens, J. F. Chemistry of Acetylenic Ethers XXXII. Formation of 1,3-Dialkyl-2-ethoxy-cyclobut- 2-ene-4-ones from Ethyl 1-Alkynyl Ethers. Recl. Trav. Chim. Pays-Bas 1958, 77, 761–768;
(c) van Daalen, J. J.; Kraak, A.; Arens, J. F. Chemistry of Acetylenic Ethers LII. Acetylenic tert-Butyl Ethers and Some Other Acetylenic Ethers with Branched Alkyl Groups: Some Remarks Concerning the Preparation and the Pyrolysis of Acetylenic Ethers. Recl. Trav. Chim. Pays-Bas 1961, 80, 810–818.
10.1002/recl.19610800802 Google Scholar
- 15 Sweis, R. F.; Schramm, M. P.; Kozmin, S. A. Silver-Catalyzed [2 + 2] Cycloadditions of Siloxy Alkynes. J. Am. Chem. Soc. 2004, 126, 7442–7443.
- 16 Zanini, M.; Cataffo, A.; Echavarren, A. M. Synthesis of Cyclobutanones by Gold(I)-Catalyzed [2 + 2] Cycloaddition of Ynol Ethers with Alkenes. Org. Lett. 2021, 23, 8989–8993.
- 17 Zhang, W.; Ready, J. M. A Concise Total Synthesis of Dictyodendrins F, H, and I Using Aryl Ynol Ethers as Key Building Blocks. J. Am. Chem. Soc. 2016, 138, 10684–10692.
- 18 Zhu, X.-Q.; Yuan, H.; Sun, Q.; Zhou, B.; Han, X.-Q.; Zhang, Z.-X.; Lu, X.; Ye, L.-W. Benign Catalysis with Zinc: Atom-Economical and Divergent Synthesis of Nitrogen Heterocycles by Formal [3 + 2] Annulation of Isoxazoles with Ynol Ethers. Green Chem. 2018, 20, 4287–4291.
- 19 Qian, H.; Sun, S.; Zhao, W.; Sun, J. An Efficient [3 + 2] Annulation for the Asymmetric Synthesis of Densely-Functionalized Pyrrolidinones and γ-Butenolides. Chem. Commun. 2020, 56, 11295–11298.
- 20 Coles-Taylor, B. L.; Ellinwood, M. L.; McCallum, M. S.; McCormack, S. L.; Jones, M. H.; Muñoz, A. G.; Michel, B. W. Access to Substituted Indenol Ethers via a Regioselective Intermolecular Carbopalladation Cascade. J. Org. Chem. 2022, 87, 16517–16525.
- 21 Minami, Y.; Shiraishi, Y.; Yamada, K.; Hiyama, T. Palladium-Catalyzed Cycloaddition of Alkynyl Aryl Ethers with Internal Alkynes via Selective Ortho C–H Activation. J. Am. Chem. Soc. 2012, 134, 6124–6127.
- 22(a) Minami, Y.; Kanda, M.; Hiyama, T. Palladium-catalyzed Cycloaddition of Alkynyl Aryl Ethers to Allenes to Form a 2,3-Bismethylidene- 2,3-dihydro-4H-1-benzopyran Framework. Chem. Lett. 2014, 43, 181–183; (b) Minami, Y.; Sakai, M.; Anami, T.; Hiyama, T. Annulation of Alkynyl Aryl Ethers with Allyl Pivalates To Give 2,3-Bismethylene- chromanes through Double C-H Bond Cleavage. Angew. Chem. Int. Ed. 2016, 55, 8701–8705.
- 23 Shen, W.-B.; Xiao, X.-Y.; Sun, Q.; Zhou, B.; Zhu, X.-Q.; Yan, J.-Z.; Lu, X.; Ye, L.-W. Highly Site Selective Formal [5 + 2] and [4 + 2] Annulations of Isoxazoles with Heterosubstituted Alkynes by Platinum Catalysis: Rapid Access to Functionalized 1,3-Oxazepines and 2,5-Dihydropyridines. Angew. Chem. Int. Ed. 2017, 56, 605–609.
- 24 Zhou, A.-H.; He, Q.; Shu, C.; Yu, Y.-F.; Liu, S.; Zhao, T.; Zhang, W.; Lu, X.; Ye, L.-W. Atom-Economic Generation of Gold Carbenes: Gold- Catalyzed Formal [3 + 2] Cycloaddition between Ynamides and Isoxazoles. Chem. Sci. 2015, 6, 1265–1271.
- 25 Coles-Taylor, B. L.; McCallum, M. S.; Lee, J. S.; Michel, B. W. Accessing 4-Oxy-Substituted Isoquinolinones via C–H Activation and Regioselective Migratory Insertion with Electronically Biased Ynol Ethers. Org. Biomol. Chem. 2018, 16, 8639–8646.
- 26 Qian, H.; Sun, J. W. Synthesis of Coumarins via [4 + 2] Cyclization of Siloxy Alkynes and Salicylaldehydes. Synlett 2021, 32, 207–210.
- 27 Suárez-Rodríguez, T.; Suárez-Sobrino, Á. L.; Ballesteros, A. Gold(I)- Catalyzed Intermolecular Formal [4 + 2] Cycloaddition of O-Aryl Ynol Ethers and Enol Ethers: Synthesis of Chromene Derivatives. Chem. - Eur. J. 2021, 27, 13079–13084.
- 28 Zhu, X.-Q.; Wang, Z.-S.; Hou, B.-S.; Zhang, H.-W.; Deng, C.; Ye, L.-W. Zinc-Catalyzed Asymmetric Formal [4 + 3] Annulation of Isoxazoles with Enynol Ethers by 6π Electrocyclization: Stereoselective Access to 2H-Azepines. Angew. Chem. Int. Ed. 2020, 59, 1666–1673.
- 29 Wender, P. A.; Ebner, C.; Fennell, B. D.; Inagaki, F.; Schröder, B. Ynol Ethers as Ketene Equivalents in Rhodium-Catalyzed Intermolecular [5 + 2] Cycloaddition Reactions. Org. Lett. 2017, 19, 5810–5813.
- 30 Zhao, W.; Wang, Z.; Sun, J. Synthesis of Eight-Membered Lactones: Intermolecular [6 + 2] Cyclization of Amphoteric Molecules with Siloxy Alkynes. Angew. Chem. Int. Ed. 2012, 51, 6209–6213.
- 31 Zhao, W.; Qian, H.; Li, Z.; Sun, J. Catalytic Ring Expansion of Cyclic Hemiaminals for the Synthesis of Medium-Ring Lactams. Angew. Chem. Int. Ed. 2015, 54, 10005–10008.
- 32 Wu, A.; Feng, Q.; Sung, H. H. Y.; Williams, I. D.; Sun, J. Synthesis of Eight-Membered Lactams through Formal [6 + 2] Cyclization of Siloxy Alkynes and Vinylazetidines. Angew. Chem. Int. Ed. 2019, 58, 6776–6780.
- 33 Santandrea, J.; Godin, E.; Collins, S. K. A Synthetic Guide to Alkynyl Sulfides. Org. Biomol. Chem. 2020, 18, 4885–4893.
- 34(a) Riddell, N.; Tam, W. Ruthenium-Catalyzed [2 + 2] Cycloadditions of Alkynyl Sulfides and Alkynyl Sulfones. J. Org. Chem. 2006, 71, 1934–1937; (b) Godoi, B.; Speranca, A.; Back, D. F.; Brandao, R.; Nogueira, C. W.; Zeni, G. Synthesis of Organochalcogen Propargyl Aryl Ethers and Their Application in the Electrophilic Cyclization Reaction: An Efficient Preparation of 3-Halo-4-Chalcogen-2H-Benzopyrans. J. Org. Chem. 2009, 74, 3469–3477; (c) Zheng, W.; Zheng, F.; Hong, Y.; Hu, L. A One-Pot Synthesis of Alkynyl Sulfides from Terminal Alkynes. Heteroat. Chem. 2012, 23, 105–110; (d) Reeves, J. T.; Camara, K.; Han, Z. S.; Xu, Y.; Lee, H.; Busacca, C. A.; Senanayake, C. H. The Reaction of Grignard Reagents with Bunte Salts: A Thiol-Free Synthesis of Sulfides. Org. Lett. 2014, 16, 1196–1199; (e) Kanemoto, K.; Yoshida, S.; Hosoya, T. Synthesis of Alkynyl Sulfides by Copper-Catalyzed Thiolation of Terminal Alkynes Using Thiosulfonates. Org. Lett. 2019, 21, 3172–3177; (f) Yang, L.; Tian, Z.-Y.; Zhang, C.-P. Transition-Metal- Free Selective Synthesis of (Z)-1,2-Diarylthio-1-arylalkenes, (2-Arylethene-1,1,2-triyl)tris(arylsulfane)s and Alkynyl Sulfides from Thiocyanates and Terminal Arylalkynes. ChemistrySelect 2019, 4, 311–315.
- 35(a) Gray, V. J.; Cuthbertson, J.; Wilden, J. D. Transition-Metal-Free Synthesis of Ynol Ethers and Thioynol Ethers via Displacement at sp Centers: A Revised Mechanistic Pathway. J. Org. Chem. 2014, 79, 5869–5874; (b) Frei, R.; Wodrich, M. D.; Hari, D. P.; Borin, P.-A.; Chauvier, C.; Waser, J. Fast and Highly Chemoselective Alkynylation of Thiols with Hypervalent Iodine Reagents Enabled through a Low Energy Barrier Concerted Mechanism. J. Am. Chem. Soc. 2014, 136, 16563–16573; (c) Chowdhury, R. M.; Wilden, J. D. An Improved Transition Metal-Free Synthesis of Arylalkynylsulfides via Substitution of a Halide at an sp-Centre. Org. Biomol. Chem. 2015, 13, 5859–5861; (d) Waldecker, B.; Kraft, F.; Golz, C.; Alcarazo, M. 5-(Alkynyl)dibenzothiophenium Triflates: Sulfur-Based Reagents for Electrophilic Alkynylation. Angew. Chem. Int. Ed. 2018, 57, 12538–12542; (e) Godin, É.; Santandrea, J.; Caron, A.; Collins, S. K. General Cu-Catalyzed Csp–S Coupling. Org. Lett. 2020, 22, 5905–5909.
- 36 Yang, Y.; Dong, W.; Guo, Y.; Rioux, R. M. Cu(I)-Catalyzed Aerobic Cross- Dehydrogenative Coupling of Terminal Alkynes with Thiols for the Construction of Alkynyl Sulfides. Green Chem. 2013, 15, 3170–3175.
- 37(a) Péna, J.; Talavera, G.; Waldecker, B.; Alcarazo, M. Alkynylthioimidazolium Salts: Efficient Reagents for the Synthesis of Alkynyl Sulfides by Electrophilic Thioalkynylation. Chem. - Eur. J. 2017, 23, 75–78; (b) Gao, W.-C.; Shang, Y.-Z.; Chang, H.-H.; Li, X.; Wei, W.-L.; Yu, X.-Z.; Zhou, R. N-Alkynylthio Phthalimide: A Shelf-Stable Alkynylthio Transfer Reagent for the Synthesis of Alkynyl Thioethers. Org. Lett. 2019, 21, 6021–6024.
- 38 Riddell, N.; Tam, W. Ruthenium-Catalyzed [2 + 2] Cycloadditions of Alkynyl Sulfides and Alkynyl Sulfones. J. Org. Chem. 2006, 71, 1934–1937.
- 39 Pounder, A.; Chen, L. D.; Tam, W. Ruthenium-Catalyzed [2 + 2] versus Homo Diels–Alder [2 + 2 + 2] Cycloadditions of Norbornadiene and Disubstituted Alkynes: A DFT Study. ACS Omega 2021, 6, 900–911.
- 40 Bai, Y.-B.; Luo, Z.; Wang, Y.; Gao, J.-M.; Zhang, L. Au-Catalyzed Intermolecular [2 + 2] Cycloadditions between Chloroalkynes and Unactivated Alkenes. J. Am. Chem. Soc. 2018, 140, 5860–5865.
- 41(a) Tornøe, C. W.; Christensen, C.; Meldal, M. Peptidotriazoles on Solid Phase: [1,2,3]-Triazoles by Regiospecific Copper(I)-Catalyzed 1,3-Dipolar Cycloadditions of Terminal Alkynes to Azides. J. Org. Chem. 2002, 67, 3057–3064;
(b) Rostovtsev, V. V.; Green, L. G.; Fokin, V. V.; Sharpless, K. B. A Stepwise Huisgen Cycloaddition Process: Copper(I)-Catalyzed Regioselective “Ligation” of Azides and Terminal Alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599.
10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4 CAS PubMed Web of Science® Google Scholar
- 42 Ding, S.; Jia, G.; Sun, J. Iridium-Catalyzed Intermolecular Azide-Alkyne Cycloaddition of Internal Thioalkynes under Mild Conditions. Angew. Chem. Int. Ed. 2014, 53, 1877–1880.
- 43(a) Destito, P.; Couceiro, J. R.; Faustino, H.; Lopez, F.; Mascareñas, J. L. Angew. Chem. Int. Ed. 2017, 56, 10766–10770; (b) Gutiérrez-González, A.; Destito, P.; Couceiro, J. R.; Pérez-González, C.; López, F.; Mascareñas, J. L. Bioorthogonal Azide–Thioalkyne Cycloaddition Catalyzed by Photoactivatable Ruthenium(II) Complexes. Angew. Chem. Int. Ed. 2021, 60, 16059–16066.
- 44 Song, W. Z.; Zheng, N.; Li, M.; Dong, K.; Li, J.-H.; Ullah, K.; Zheng, Y.-B. Regiodivergent Rhodium(I)-Catalyzed Azide-Alkyne Cycloaddition (RhAAC) to Access Either Fully Substituted Sulfonyl-1,2,3-triazoles under Mild Conditions. Org. Lett. 2018, 20, 6705– 6709.
- 45(a) Song, W.; Zheng, N.; Li, M.; He, J.; Li, J.; Dong, K.; Ullah, K.; Zheng, Y. Rhodium(I)-Catalyzed Regioselective Azide-internal Alkynyl Trifluoromethyl Sulfide Cycloaddition and Azide-internal Thioalkyne Cycloaddition under Mild Conditions. Adv. Synth. Catal. 2019, 361, 469–475; (b) Song, W.-Z.; Li, M.; Dong, K.; Zheng, Y.-B. Ruthenium- Catalyzed Highly Regioselective Azide-Internal Thiocyanatoalkyne Cycloaddition under Mild Conditions: Experimental and Theoretical Studies. Adv. Synth. Catal. 2019, 361, 5258–5263; (c) Li, M.; Dong, K.; Zheng, Y.-B.; Song, W.-Z. Copper-Catalyzed Cascade Click/Nucleophilic Substitution Reaction to Access Fully Substituted Triazolyl-Organosulfurs. Org. Biomol. Chem. 2019, 17, 9933–9941.
- 46 Racine, S.; Hegedüs, B.; Scopelliti, R.; Waser, J. Divergent Reactivity of Thioalkynes in Lewis Acid Catalyzed Annulations with Donor–Acceptor Cyclopropanes. Chem. - Eur. J. 2016, 22, 11997–12001.
- 47 Reddy, R. J.; Ball-Jones, M. P.; Davies, P. W. Alkynyl Thioethers in Gold-Catalyzed Annulations To Form Oxazoles. Angew. Chem. Int. Ed. 2017, 56, 13310–13313.
- 48 Feng, Q.; Huang, H.; Sun, J. Ru-Catalyzed [3 + 2] Cycloaddition of Nitrile Oxides and Electron-Rich Alkynes with Reversed Regioselectivity. Org. Lett. 2021, 23, 2431–2436.
- 49 Simm, P. E.; Sekar, P.; Richardson, J.; Davies, P. W. Gold(I)-Catalyzed Synthesis of 3-Sulfenyl Pyrroles and Indoles by a Regioselective Annulation of Alkynyl Thioethers. ACS Catal. 2021, 11, 6357–6362.
- 50 More, S. A.; Sadaphal, V. A.; Kuo, T.-C.; Cheng, M.-J.; Liu, R.-S. Reactions of Thioalkynes with Diarylketenes via [3 + 2]-Annulation versus Benzannulation Using Au and P(C6F5)3 Catalysts. Chem. Commun. 2022, 58, 10064–10067.
- 51 Hilt, G.; Lüers, S.; Harms, K. The First Broad Application of Alkynyl Sulfides as Dienophiles in Cobalt(I)-Catalyzed Diels–Alder Reactions. J. Org. Chem. 2004, 69, 624–630.
- 52
Aoyagi, S.; Ohata, S.; Shimada, K.; Takikawa, Y. Yb(OTf)3-Catalyzed [4 + 2] Cycloaddition of Allenyltrimethylsilylthioketenes with Arylaldimines. Synlett 2007, 615–618.
10.1055/s-2007-970748 Google Scholar
- 53 Bu, H.-Z.; Li, H.-H.; Luo, W.-F.; Luo, C.; Qian, P.-C.; Ye, L.-W. Synthesis of 2H-Chromenes via Unexpected [4 + 2] Annulation of Alkynyl Thioethers with o-Hydroxybenzyl Alcohols. Org. Lett. 2020, 22, 648–652.




