Visible Light-Promoted Aerobic Oxidation of α-Silyl Styrenes with Alcohols
Yan Tan
College of Chemistry, Zhengzhou University, Zhengzhou, Henan, 450001 China
Search for more papers by this authorBo Yang
Department of Chemistry, Zhejiang University, Hangzhou, Zhejiang, 310058 China
Search for more papers by this authorJiale Ying
Department of Chemistry, Zhejiang University, Hangzhou, Zhejiang, 310058 China
Search for more papers by this authorBing Yu
College of Chemistry, Zhengzhou University, Zhengzhou, Henan, 450001 China
Search for more papers by this authorCorresponding Author
Zhan Lu
College of Chemistry, Zhengzhou University, Zhengzhou, Henan, 450001 China
Department of Chemistry, Zhejiang University, Hangzhou, Zhejiang, 310058 China
E-mail: [email protected]Search for more papers by this authorYan Tan
College of Chemistry, Zhengzhou University, Zhengzhou, Henan, 450001 China
Search for more papers by this authorBo Yang
Department of Chemistry, Zhejiang University, Hangzhou, Zhejiang, 310058 China
Search for more papers by this authorJiale Ying
Department of Chemistry, Zhejiang University, Hangzhou, Zhejiang, 310058 China
Search for more papers by this authorBing Yu
College of Chemistry, Zhengzhou University, Zhengzhou, Henan, 450001 China
Search for more papers by this authorCorresponding Author
Zhan Lu
College of Chemistry, Zhengzhou University, Zhengzhou, Henan, 450001 China
Department of Chemistry, Zhejiang University, Hangzhou, Zhejiang, 310058 China
E-mail: [email protected]Search for more papers by this authorComprehensive Summary
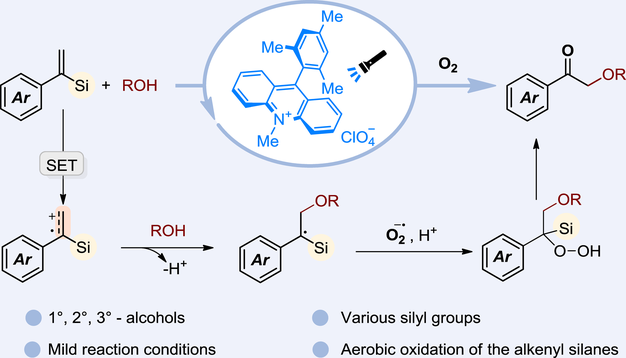
A mechanistically distinctive visible-light-promoted metal-free aerobic oxidation of alkenyl silanes with alcohols was disclosed to efficiently construct α-alkoxy ketones under mild conditions. The primary, secondary, and tertiary alcohols could be used as reactants. The protocol could be carried out on a gram-scale. Various derivatizations of products could be conducted. Mechanistic studies indicated the reaction was initiated by single-electron oxidation of the alkenyl silanes, rather than radical addition to alkenyl silanes.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202400602-sup-0001-supinfo.pdfPDF document, 11.7 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Colpaert, F.; Mangelinckx, S.; Rocchetti, M. T.; De Kimpe, N. New General Synthesis of α-alkoxyketones via α'-alkylation, α-alkylation and α, α'-dialkylation of α-alkoxyketimines. Org. Biomol. Chem. 2011, 9, 549–558.
- 2(a) Shevchenko, G. A.; Oppelaar, B.; List, B. An Unexpected α-Oxidation of Cyclic Ketones with 1,4-Benzoquinone by Enol Catalysis. Angew. Chem. Int. Ed. 2018, 57, 10756–10759; (b) Zhu, C.-J.; Zhang, Y.-F.; Zhao, H.-Q.; Huang, S.-J.; Zhang, M.; Su, W.-P. Sodium Iodide-Catalyzed Direct α-Alkoxylation of Ketones with Alcohols Oxidation of α-Iodo Ketone Intermediates. Adv. Synth. Catal. 2015, 357, 331–338; (c) Yu, H.; Xu, Y.-L.; Fang, Y.; Dong, R. Direct Synthesis of α-Alkoxy Ketones by Oxidative C-O Bond Formation. Eur. J. Org. Chem. 2016, 2016, 5257–5262; (d) Wu, X.; Song, S.-G.; Zhang, X.; Fu, Y.-M.; Zhu, C.-F.; Li, Y.-G. Copper-Catalyzed Direct Oxidative α-Alkoxylation of 4-Isochromanones. Eur. J. Org. Chem. 2021, 2021, 2436–2439; (e) Uyanik, M.; Okamoto, H.; Yasui, T.; Ishihara, K. Quaternary Ammonium (hypo) Iodite Catalysis for Enantioselective Oxidative Cycloetherification. Science 2010, 328, 1376–1379.
- 3(a) Banoun, C.; Bourdreux, F.; Magnier, E.; Dagousset, G. Intermolecular C-O Bond Formation with Alkoxyl Radicals: Photoredox-Catalyzed α-Alkoxylation of Carbonyl Compounds. Org. Lett. 2021, 23, 8926–8930; (b) Mizar, P.; Wirth, T. Flexible Stereoselective Functionalizations of Ketones through Umpolung with Hypervalent Iodine Reagents. Angew. Chem. Int. Ed. 2014, 53, 5993–5997.
- 4(a) Wu, F.; Guo, Y.; Ren, Z.-H.; Chen, Z.-X.; Liu, X.-Q.; Wang, C.; Rong, L.-C. Electrochemical Radical Reactions of Enol Acetates and Free Alcohols Directly Access to α-Alkoxylated Carbonyl Compounds. J. Org. Chem. 2023, 88, 8825–8834; (b) Zhang, P.; Ma, J.-W.; Liu, X.; Xue, F.; Zhang, Y.-H.; Wang, B.; Jin, W.-W.; Xia, Y.; Liu, C.-J. Electrochemical Synthesis of α-Thiocyanated/Methoxylated Ketones Using Enol Acetates. J. Org. Chem. 2023, 88, 16122–16131.
- 5(a) Nair, V.; Nair, L. G.; Panicker, S. B.; Sheeba, V.; Augustine, A. Novel Cerium(IV) Ammonium Nitrate Mediated Transformation of Styrenes to α-Methoxy Acetophenones. Chem. Lett. 2000, 29, 584–585;
10.1246/cl.2000.584 Google Scholar(b) Asano, Y.; Nagasawa, Y.; Yamaguchi, E.; Itoh, A. Aerobic Photooxidative Synthesis of β-Alkoxy Monohydroperoxides Using an Organo Photoredox Catalyst Controlled by a Base. Chem. Asian J. 2018, 13, 409–412. For the synthesis of α-ester ketones from styrenes, see: (c) Zhang, Q.-B.; Ban, Y.-L.; Zhou, D.-G.; Zhou, P.-P.; Wu, L.-Z.; Liu, Q. Preparation of α-Acyloxy Ketones via Visible-Light-Driven Aerobic Oxo-Acyloxylation of Olefins with Carboxylic Acids. Org. Lett. 2016, 18, 5256–5259; (d) Zhang, S.-Z; Zhang, J.-Q; Zou, H.-B. Pd-Catalyzed TBHP-Mediated Selective Wacker-Type Oxidation and Oxo-acyloxylation of Olefins Using a 2-(1H-Indazol-1-yl)quinoline Ligand. Org. Lett. 2023, 25, 1850–1855.
- 6(a) Luh, T.-Y.; Liu, S.-T. Synthetic Applications of Allylsilanes and Vinylsilanes. In The Chemistry of Organic Silicon Compounds, Vol. 2, Eds.: Z. Rappoport; Y Apeloig, Wiley, Weinheim, Germany, 1998, pp. 1793–1868;
10.1002/0470857250.ch30 Google Scholar(b) Kustiana, B. A.; Melen, R. L.; Morrill, L. C. One-Pot Synthesis of Styrene Derivatives from Allyl Silanes via B(C6F5)3-Catalyzed Isomerization-Hiyama Coupling. Org. Lett. 2022, 24, 8694–8697 and its references; (c) Wang, X.; Cheng, Z.-Y.; Lu, Z. Hydrosilylation of C-C π-Bonds. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering, Elsevier, 2023.10.1016/B978-0-323-96025-0.00015-6 Google Scholar
- 7(a) Fristad, W. E.; Bailey, T. R.; Paquette, L. A. Regiospecific Photosensitized Oxygenation of Vinylsilanes. A Method for Converting Saturated Ketones to 1,2-Transposed Allylic Alcohols. Possible Role of Silicon in Directing the Regioselectivity of Epoxysilane Cleavage Reactions. J. Am. Chem. Soc. 1979, 101, 4420–4423;
(b) Adam, W.; Richter, M. J. Highly Regio- and Diastereoselective One-Pot Synthesis of Silyl Epoxy Alcohols and Vinylsilanes by Direct Hydroxy-Epoxidation. J. Org. Chem. 1994, 59, 3341–3346;
(c) Adam, W.; Richter, M. J. Regioselectivity of the Singlet Oxygen Ene Reaction (Schenck Reaction) with Vinylsilanes. J. Org. Chem. 1994, 59, 3335–3340;
(d) Adam, W.; Richter, M. J. One pot synthesis of α-trimethylsilyl enones from vinyl silanes. Synthesis 1994, 176–180.
10.1055/s-1994-25433 Google Scholar
- 8 Nakatani, S.; Yoshida, J.; Isoe, S. Electra-Initiated Oxygenation of Alkenylsilanes in the Presence of Thiophenol. Tetrahedron 1993, 49, 2011–2024.
- 9 Kondo, J.; Shinokubo, H.; Oshima, K. From alkenylsilanes to ketones with air as the oxidant. Angew. Chem. Int. Ed. 2003, 42, 825–827.
- 10(a) Cheng, X.-K.; Hu, X.-G.; Lu, Z. Visible-Light-Promoted Aerobic Homogenous Oxygenation Reactions. Chin. J. Org. Chem. 2017, 37, 251–266; (b) Stahl, S. S. Palladium-Catalyzed Oxidation of Organic Chemicals with O2. Science 2005, 309, 1824-1826; (c) Liang, Y.-J.; Wei, J. L.; Qiu, X.; Jiao, N. Homogeneous Oxygenase Catalysis. Chem. Rev. 2018, 118, 4912–4945; (d) Liu, J.; Guðmundsson, A.; Bäckvall, J.-E. Efficient Aerobic Oxidation of Organic Molecules by Multistep Electron Transfer. Angew. Chem. Int. Ed. 2021, 60, 15686–15704.
- 11(a) Roth, H. G., Romero, N. A.; Nicewicz, D. A. Experimental and Calculated Electrochemical Potentials of Common Organic Molecules for Applications to Single-Electron Redox Chemistry. Synlett 2016, 27, 714–723; (b) Xiao, Z.-L.; Xie, Z.-Z.; Yuan, C.-P.; Deng, K.-Y.; Chen, K.; Chen, H.-B.; Xiang, H.-Y.; Yang, H. Photosensitized 1,2-Difunctionalization of Alkenes to Access β-Amino Sulfonamides. Org. Lett. 2024, 26, 2108–2113.
- 12(a) Ohkubo, K.; Mizushima, K.; Iwata, R.; Souma, K.; Suzuki, N.; Fukuzumi, S. Simultaneous Production of p-Tolualdehyde and Hydrogen Peroxide in Photocatalytic Oxygenation of p-Xylene and Reduction of Oxygen with 9-Mesityl-10-methylacridinium Ion Derivatives. Chem Commun. 2010, 46, 601–603; (b) Fukuzumi, S.; Kotani, H.; Ohkubo, K.; Ogo, S.; Tkachenko, N. V.; Lemmetyinen, H. Electron-Transfer State of 9-Mesityl-10-methylacridinium Ion with a Much Longer Lifetime and Higher Energy Than That of the Natural Photosynthetic Reaction Center. J. Am. Chem. Soc. 2004, 126, 1600–1601; (c) Fukuzumi, S.; Ohkubo, K.; Suenobu, T.; Kato, K.; Fujitsuka, M.; Ito, O. Photoalkylation of 10-Alkylacridinium Ion via a Charge-Shift Type of Photoinduced Electron Transfer Controlled by Solvent Polarity. J. Am. Chem. Soc. 2001, 123, 8459–8467.
- 13(a) Hamilton, D. S.; Nicewicz, D. A. Direct Catalytic Anti-markovnikov Hydroetherification of Alkenols. J. Am. Chem. Soc. 2012, 134, 18577–18580;
(b) Asano, Y.; Nagasawa, Y.; Yamaguchi, E.; Itoh, A. Aerobic Photooxidative Synthesis of β-Alkoxy Monohydroperoxides Using an Organo Photoredox Catalyst Controlled by a Base. Chem. Asian J. 2018, 13, 409–412;
(c) Yang, B.; Lu, Z. Visible light-promoted dihydroxylation of styrenes with water and dioxygen. Chem. Commun. 2017, 53, 12634–12637;
(d) Cao, M.-Y.; Ren, X.; Lu, Z. Olefin Difunctionalizations via Visible Light Photocatalysis. Tetrahedron Lett. 2015, 56, 3732–3742;
(e) Wu, X.-Q; Li, H.-Y; He, F.; Qu, J.-P; Chen, Y.-F. Nickel/Quinim Enabled Asymmetric Carbamoyl-Acylation of Unactivated Alkenes. Chin. J. Chem. 2023, 41, 1673–1678;
10.1002/cjoc.202200856 Google Scholar(f) Wang, L.; Zhang, H.; Zhu, C.; Feng, C. Expedient Trifluoromethylacylation of Styrenes Enabled by Photoredox Catalysis. Chin. J. Chem. 2021, 40, 59–6410.1002/cjoc.202100599 Google Scholar
- 14(a) Inoue, A.; Kondo, J.; Shinokubo, H.; Oshima, K. Facile Synthesis of Acylsilanes via Aerobic Oxidation of gem-Disilylalkylcopper Compounds. J. Am. Chem. Soc. 2001, 123, 11109–11110.




