Enantioselective Total Synthesis of (+)-Propolisbenzofuran B†
Wen-Xiu Xu
Department of Chemistry, Zhejiang University, 866 Yuhangtang Road, Hangzhou, Zhejiang, 310058 China
Key Laboratory of Precise Synthesis of Functional Molecules of Zhejiang Province, Department of Chemistry and Research Center for Industries of the Future, Westlake University, 600 Dunyu Road, Hangzhou, Zhejiang, 310030 China
Search for more papers by this authorLi-Han Zhao
Key Laboratory of Precise Synthesis of Functional Molecules of Zhejiang Province, Department of Chemistry and Research Center for Industries of the Future, Westlake University, 600 Dunyu Road, Hangzhou, Zhejiang, 310030 China
Search for more papers by this authorYao Zhu
Key Laboratory of Precise Synthesis of Functional Molecules of Zhejiang Province, Department of Chemistry and Research Center for Industries of the Future, Westlake University, 600 Dunyu Road, Hangzhou, Zhejiang, 310030 China
Search for more papers by this authorCorresponding Author
Hai-Hua Lu
Key Laboratory of Precise Synthesis of Functional Molecules of Zhejiang Province, Department of Chemistry and Research Center for Industries of the Future, Westlake University, 600 Dunyu Road, Hangzhou, Zhejiang, 310030 China
Institute of Natural Sciences, Westlake Institute for Advanced Study, 18 Shilongshan Road, Hangzhou, Zhejiang, 310024 China
E-mail: [email protected]Search for more papers by this authorWen-Xiu Xu
Department of Chemistry, Zhejiang University, 866 Yuhangtang Road, Hangzhou, Zhejiang, 310058 China
Key Laboratory of Precise Synthesis of Functional Molecules of Zhejiang Province, Department of Chemistry and Research Center for Industries of the Future, Westlake University, 600 Dunyu Road, Hangzhou, Zhejiang, 310030 China
Search for more papers by this authorLi-Han Zhao
Key Laboratory of Precise Synthesis of Functional Molecules of Zhejiang Province, Department of Chemistry and Research Center for Industries of the Future, Westlake University, 600 Dunyu Road, Hangzhou, Zhejiang, 310030 China
Search for more papers by this authorYao Zhu
Key Laboratory of Precise Synthesis of Functional Molecules of Zhejiang Province, Department of Chemistry and Research Center for Industries of the Future, Westlake University, 600 Dunyu Road, Hangzhou, Zhejiang, 310030 China
Search for more papers by this authorCorresponding Author
Hai-Hua Lu
Key Laboratory of Precise Synthesis of Functional Molecules of Zhejiang Province, Department of Chemistry and Research Center for Industries of the Future, Westlake University, 600 Dunyu Road, Hangzhou, Zhejiang, 310030 China
Institute of Natural Sciences, Westlake Institute for Advanced Study, 18 Shilongshan Road, Hangzhou, Zhejiang, 310024 China
E-mail: [email protected]Search for more papers by this author† Dedicated to the Special Issue of Emerging Investigators in 2024.
Comprehensive Summary
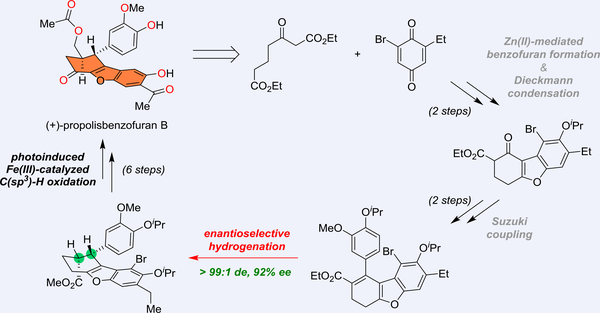
The first catalytic asymmetric total synthesis of (+)-propolisbenzofuran B, enabled by a highly enantioselective rhodium-catalyzed hydrogenation of a tetrasubstituted olefin, was described. Other noteworthy aspects include the construction of the central hydrodibenzo[b,d]furan core through a sequence of Zn(II)-mediated regioselective benzofuran formation and Dieckmann condensation, as well as C-H oxidations, involving a visible light-induced Fe(III)-catalyzed benzylic C(sp3)-H oxidation. Additionally, the absolute configuration was confirmed by X-ray analysis of a carbonate intermediate.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202400563-sup-0001-supinfo.pdfPDF document, 2.8 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For selected reviews, see: (a) Heravi, M. M.; Zadsirjan, V.; Hamidi, H.; Tabar Amiri, P. H. Total Synthesis of Natural Products Containing Benzofuran Rings. RSC Adv. 2017, 7, 24470–24521; (b) Miao, Y.; Hu, Y.; Yang, J.; Liu, T.; Sun, J.; Wang, X. Natural Source, Bioactivity and Synthesis of Benzofuran Derivatives. RSC Adv. 2019, 9, 27510–27540.
- 2 Liu, Y.; Kubo, M.; Fukuyama, Y. Nerve Growth Factor-Potentiating Benzofuran Derivatives from the Medicinal Fungus Phellinus ribis. J. Nat. Prod. 2012, 75, 2152−2157.
- 3 Wang, W.; Wang, L.; Huang, X.; Jiang, R.; Yang, X.; Zhang, D.; Chen, W.; Tang, B.; Wang, Y.; Zhang, X.; Ye, W. Two Pairs of New Benzofuran Enantiomers with Unusual Skeletons from Eupatorium Chinense. Tetrahedron Lett. 2013, 54, 3321−3324.
- 4 Micheal, E.; Anton, D. US005846995A, 1998-12-08.
- 5 Banskota, A.; Tezuka, Y.; Midorikawa, K.; Matsushige, K.; Kadota, S. Two Novel Cytotoxic Benzofuran Derivatives from Brazilian Propolis. J. Nat. Prod. 2000, 63, 1277−1279.
- 6For selected examples, see: (a) Takeya, T.; Kondo, H.; Otsuka, T.; Tomita, K.; Okamoto, I.; Tamura, O. A Novel Construction of Dibenzofuran-1,4-Diones by Oxidative Cyclization of Quinone-Arenols. Org. Lett. 2007, 9, 2807–2810; (b) Phun, L. H.; Patil, D. V.; Cavitt, M. A.; France, S. A Catalytic Homo-Nazarov Cyclization Protocol for the Synthesis of Heteroaromatic Ring-Fused Cyclohexanones. Org. Lett. 2011, 13, 1952–1955; (c) Xiao, B. X.; Du, W.; Chen, Y. C. Asymmetric Dearomatizative Diels–Alder Reaction for the Construction of Hydrodibenzo[b,d]Furan Frameworks with Tetrasubstituted Stereogenic Centers. Adv. Synth. Catal. 2017, 359, 1018–1027; (d) Wang, Y.; Lin, J. B.; Xie, J. K.; Lu, H.; Hu, X. Q.; Xu, P. F. Dearomative Dienolate-Mediated Catalysis: A Remote Activation Strategy for Asymmetric Functionalization of Benzylic C–H Bonds of Heteroaryl Aldehydes. Org. Lett. 2018, 20, 5835–5839; (e) He, Z. L.; Chen, P.; Chen, Z. C.; Du, W.; Chen, Y. C. Construction of Hydrodibenzo[b,d]furan Frameworks from Morita–Baylis–Hillman Carbonates of Isatins and o-Hydroxy Enones via Palladium and Brønsted Base Relay Catalysis. Org. Lett. 2022, 24, 100– 104; (f) Hu, N.; Chen, Y.; Zhang, Y. Q.; Lan, P.; Hu, Y. J.; Ward, J. S.; Gardiner, M. G.; Banwell, M. G. A Gem-Dibromocyclopropane Ring-Expansion/Mizoroki-Heck Cyclisation Route to Tetrahydrodibenzofurans. Eur. J. Org. Chem. 2024, 27, e202400038.
- 7For selected examples, see: (a) Liou, J. P.; Cheng, C. Y. Total Synthesis of (±)-Desoxycodeined: a Novel Route to the Morphine Skeleton. Tetrahedron Lett. 2000, 41, 915–918; (b) Yamashita, M.; Ohta, N.; Shimizu, T.; Matsumoto, K.; Matsuura, Y.; Kawasaki, I.; Tanaka, T.; Maezaki, N.; Ohta, S. First Total Synthesis of (±)-Linderol A, a Tricyclic Hexahydrodibenzofuran Constituent of Lindera Umbellata Bark, with Potent Inhibitory Activity on Melanin Biosynthesis of Cultured B-16 Melanoma Cells. J. Org. Chem. 2003, 68, 1216–1224; (c) Yamashita, M.; Yadav, N. D.; Sawaki, T.; Takao, I.; Kawasaki, I.; Sugimoto, Y.; Miyatake, A.; Murai, K.; Takahara, A.; Kurume, A.; Ohta, S. Asymmetric Total Synthesis of (−)-Linderol A. J. Org. Chem. 2007, 72, 5697–5703; (d) Zhang, C.; Liu, J.; Du, Y. Total Synthesis of Ribisin A. Tetrahedron Lett. 2014, 55, 959–961; (e) Lan, P.; Banwell, M. G.; Ward, J. S.; Willis, A. C. Chemoenzymatic Total Synthesis and Reassignment of the Absolute Configuration of Ribisin C. Org. Lett. 2014, 16, 228–231; (f) Boyd, D. R.; Sharma, N. D.; McGivern, C. J.; Stevenson, P. J.; Hoering, P.; Allen, C. C. R. Chemoenzymatic Synthesis of (−)-Ribisins A and B from Dibenzo[b,d]furan. J. Org. Chem. 2019, 84, 15165–15172; (g) Tan, S. H.; Karuppasamy, M.; Lan, P.; Zhang, Y.; Hu, J.; Lai, X.; Siok-Cheng Lim, B.; Liu, W.; Chen, J.; Chew, E. H.; Banwell, M. G. Ribisins and Certain Analogues Exert Neuroprotective Effects through Activation of the Keap1-Nrf2-ARE Pathway. ChemMedChem 2022, 17, e202200292; (h) Zhang, Y.; Lan, P.; Banwell, M. G. Synthetic Studies Concerned with the Proposed Structure of Ribisin F, a Compound with Nerve Growth Factor Potentiating Activity Isolated from the Medicinal Fungus Phellinus Ribis. Eur. J. Org. Chem. 2023, 26, e202300010.
- 8(a) Ghisalberti, E. L. Propolis: A Review. Bee World 1979, 60, 59–84; (b) Burdock, G. A. Review of the Biological Properties and Toxicity of Bee Propolis (Propolis). Food Chem. Toxicol. 1998, 36, 347–363; (c) Simone-Finstrom, M.; Spivak, M. Propolis and Bee Health: The Natural History and Significance of Resin Use by Honey Bees. Apidologie 2010, 41, 295–311.
- 9 Marcucci, M. C. Propolis: chemical composition, biological properties and therapeutic activity. Apidologie 1995, 26, 83–99.
- 10(a) Jones, B.; Avetta, C.; Thomson, R. J. Total Synthesis of Propolisbenzofuran B. Chem. Sci. 2014, 5, 1794–1798; (b) Srinivas, K.; Ramana, C. Total Synthesis of Propolisbenzofuran B. Org. Lett. 2017, 19, 6466–6469; (c) Sen, B.; Bera, M.; Singh, M. S.; Hajra, S. Asymmetric total Synthesis of (+)-Propolisbenzofuran B. Chem. Commun. 2023, 59, 8254−8257.
- 11(a) Corey, E. J.; Cheng, X.-M. The Logic of Chemical Synthesis, Wiley, 1989;
(b) Hoffmann, R. W. Elements of Synthesis Planning, Springer, 2009;
10.1007/978-3-540-79220-8 Google Scholar(c) Warren, S.; Wyatt, P. Organic Synthesis: The Disconnection Approach, 2nd Ed., Wiley-VCH, 2011.
- 12(a) Newhouse, T.; Baran, P. S.; Hoffmann, R. W. The Economies of Synthesis. Chem. Soc. Rev. 2009, 38, 3010–3021; (b) Burns, N. Z.; Baran, P. S.; Hoffmann, R. W. Redox Economy in Organic Synthesis. Angew. Chem. Int. Ed. 2009, 48, 2854–2867; (c) Trost, B. M. The At-om Economy−A Search for Synthetic Efficiency. Science 1991, 254, 1471−1477; (d) Wender, P. A. Toward the Ideal Synthesis and Trans-formative Therapies: The Roles of Step Economy and Function Oriented Synthesis. Tetrahedron 2013, 69, 7529−7550; (e) Wilson, R. M.; Danishefsky, S. J. Pattern Recognition in Retrosynthetic Analysis: Snapshots in Total Synthesis. J. Org. Chem. 2007, 72, 4293−4305; (f) Hayashi, Y. Pot Economy and One-Pot Synthesis. Chem. Sci. 2016, 7, 866−880; (g) Hayashi, Y. Time Economy in Total Synthesis. J. Org. Chem. 2021, 86, 1−23.
- 13(a) Hoffmann, R. W. Protecting-Group-Free Synthesis. Synthesis 2006, 3531–3541;
(b) Young, I. S.; Baran, P. S. Protecting-Group-Free Synthesis as an Opportunity for Invention. Nat. Chem. 2009, 1, 193−205;
(c) Saicic, R. N. Protecting Group-Free Syntheses of Natural Products and Biologically Active Compounds. Tetrahedron 2014, 70, 8183–8218;
(d) Hui, C.; Chen, F.; Pu, F.; Xu, J. Innovation in Protecting-Group-Free Natural Product Synthesis. Nat. Rev. Chem. 2019, 3, 85–107;
(e) Fernandes, R. A.; Kumar, P.; Choudhary, P. Advances in Catalytic and Protecting-Group-Free Total Synthesis of Natural Products: A Recent Update. Chem. Commun. 2020, 56, 8569–8590;
(f) Fernandes, R. A.; Kumar, P.; Choudhary, P. Evolution of Strategies in Protecting-Group-Free Synthesis of Natural Products: A Recent Update. Eur. J. Org. Chem. 2021, 711–740;
10.1002/ejoc.202001246 Google Scholar(g) Protecting-Group-Free Organic Synthesis: Improving Economy and Efficiency, Ed.: R. A. Fernandes, John Wiley & Sons, Hoboken, NJ, 2018.10.1002/9781119295266 Google Scholar
- 14(a) Hendrickson, J. B. Systematic Synthesis Design. IV. Numerical Codification of Construction Reactions. J. Am. Chem. Soc. 1975, 97, 5784−5800; (b) Gaich, T.; Baran, P. S. Aiming for the Ideal Synthesis. J. Org. Chem. 2010, 75, 4657−4673; (c) Peters, D. S.; Pitts, C. R.; McClymont, K. S.; Stratton, T. P.; Bi, C.; Baran, P. S. Ideality in Context: Motivations for Total Synthesis. Acc. Chem. Res. 2021, 54, 605−617.
- 15 Doering, N. A.; Sarpong, R.; Hoffmann, R. W. A Case for Bond-Network Analysis in the Synthesis of Bridged Polycyclic Complex Molecules: Hetidine and Hetisine Diterpenoid Alkaloids. Angew. Chem. Int. Ed. 2020, 59, 10722–10731.
- 16(a) Cao, M.-Y.; Ma, B.-J.; Gu, Q.-X.; Fu, B.; Lu, H.-H. Concise Enantioselective Total Synthesis of Daphenylline Enabled by an Intramolecular Oxidative Dearomatization. J. Am. Chem. Soc. 2022, 144, 5750−5755; (b) Xu, W.-X.; Peng, Z.; Gu, Q.-X.; Zhu, Y.; Zhao, L.-H.; Lu, H.-H. Cyclolignan Synthesis Streamlined by Enantioselective Hydrogenation of Tetrasubstituted Olefins. Nat. Syn. 2024, 3, DOI: https://doi.org/10.1038/s44160-024-00564-y.
- 17(a) Lin, Z.; Tong, L.; Qiu, H.; Li, Z.; Shen, L.; Che, N.; Yu, X. The Synthesis of 5-Hydroxybenzofurans via Tandem in situ Oxidative Coupling and Cyclization. SynOpen 2022, 6, 158–163; (b) Craven, P. G. E.; Taylor, R. J. K. Total synthesis and structural confirmation of (±)-cuevaene A. Tetrahedron Lett. 2012, 53, 5422–5425.
- 18 Narayanam, J. M. R.; Tucker, J. W.; Stephenson, C. R. J. Electron-Transfer Photoredox Catalysis: Development of a Tin-Free Reductive Dehalogenation Reaction. J. Am. Chem. Soc. 2009, 131, 8756–8757.
- 19 Luo, S.; Zificsak, C. A.; Hsung, R. P. Intramolecular Formal Aza-[3 + 3] Cycloaddition Approach to Indoloquinolizidine Alkaloids. A Stereoselective Total Synthesis of (±)-Tangutorine Org. Lett. 2003, 5, 4709–4712.
- 20 Venturi, F.; Venturi, C.; Liguori, F.; Cacciarini, M.; Montalbano, M.; Nativi, C. A New Scaffold for the Stereoselective Synthesis of α-O-Linked Glycopeptide Mimetics J. Org. Chem. 2004, 69, 6153–6155.
- 21(a) Noyori, R. Asymmetric Catalysis in Organic Synthesis, Wiley, New York, 1994; (b) Kitamura, M.; Noyori, R. Ruthenium in Organic Synthesis, Ed.: S.-I. Murahashi, Wiley-VCH, Weinheim, 2005, pp. 3–52; (c) Chi, Y.; Tang, W.; Zhang, X. In Modern Rhodium-Catalyzed Organic Reactions, Ed.: P. A. Evans, Wiley-VCH, Weinheim, 2005, pp. 1–31; (d) Roseblade, S. J.; Pfaltz, A. Iridium-Catalyzed Asymmetric Hydrogenation of Olefins. Acc. Chem. Res. 2007, 40, 1402–1411; (e) Verendel, J. J.; Pàmies, O.; Diéguez, M.; Andersson, P. G. Asymmetric Hydrogenation of Olefins using Chiral Crabtree-Type Catalysts: Scope and Limitations. Chem. Rev. 2014, 114, 2130–2169; (f) Chen, Q.-A.; Ye, Z.-S.; Duan, Y.; Zhou, Y.-G. Homogeneous Palladium-Catalyzed Asymmetric Hydrogenation. Chem. Soc. Rev. 2013, 42, 497–511; (g) Zhang, Z.; Butt, N. A.; Zhang, W. Asymmetric Hydrogenation of Nonaromatic Cyclic Substrates. Chem. Rev. 2016, 116, 14769–14827; (h) Wen, J.; Wang, F.; Zhang, X. Asymmetric Hydrogenation Catalyzed by First-Row Transition Metal Complexes. Chem. Soc. Rev. 2021, 50, 3211–3237; (i) Welch, G. C.; San Juan, R. R.; Masuda, J. D.; Stephan, D. W. Reversible, Metal-Free Hydrogen Activation. Science 2006, 314, 1124–1126; (j) Meng, W.; Feng, X.; Du, H. Lewis Pairs Catalyzed Asymmetric Metal-Free Hydrogenations and Hydrosilylations. Acc. Chem. Res. 2018, 51, 191–201.
- 22(a) Kraft, S.; Ryan, K.; Kargbo, R. B. Recent Advances in Asymmetric Hydrogenation of Tetrasubstituted Olefins. J. Am. Chem. Soc. 2017, 139, 11630–11641; (b) Margarita, C.; Andersson, P. G. Evolution and Prospects of the Asymmetric Hydrogenation of Unfunctionalized Olefins. J. Am. Chem. Soc. 2017, 139, 1346–1356.
- 23For selected examples, see: (a) Zhao, Q.-K.; Wu, X.; Li, L.-P.; Yang, F.; Xie, J.-H.; Zhou, Q.-L. Asymmetric Hydrogenation of β-Aryl Alkylidene Malonate Esters: Installing an Ester Group Significantly Increases the Efficiency. Org. Lett. 2021, 23, 1675–1680; (b) Molinaro, C.; Shultz, S.; Roy, A.; Lau, S.; Trinh, T.; Angelaud, R.; O’Shea, P.; Abele, S.; Cameron, M.; Corley, E. A Practical Synthesis of Renin Inhibitor MK-1597 (ACT-178882) via Catalytic Enantioselective Hydrogenation and Epimerization of Piperidine Intermediate. J. Org. Chem. 2011, 76, 1062–1071; (c) Christensen, M.; Nolting, A.; Shevlin, M.; Weisel, M.; Maligres, P. E.; Lee, J.; Orr, R. K.; Plummer, C. W.; Tudge, M. T.; Campeau, L. C.; Ruck, R. T. Enantioselective Synthesis of α-Methyl-β-Cyclopropyldihydro-Cinnamates. J. Org. Chem. 2016, 81, 824–830; (d) Ponra, S.; Rabten, W.; Yang, J.; Wu, H.; Kerdphon, S.; Andersson, P. G. Diastereo- and Enantioselective Synthesis of Fluorine Motifs with Two Contiguous Stereogenic Centers. J. Am. Chem. Soc. 2018, 140, 13878–13883; (e) Kerdphon, S.; Ponra, S.; Yang, J.; Wu, H.; Eriksson, L.; Andersson, P. G. Diastereo- and Enantioselective Synthesis of Structurally Diverse Succinate, Butyrolactone, and Trifluoromethyl Derivatives by Iridium-Catalyzed Hydrogenation of Tetrasubstituted Olefins. ACS Catal. 2019, 9, 6169–6176; (f) Liu, Y.-T.; Chen, J.-Q.; Li, L.-P.; Shao, X.-Y.; Xie, J.-H.; Zhou, Q.-L. Asymmetric Hydrogenation of Tetrasubstituted Cyclic Enones to Chiral Cycloalkanols with Three Contiguous Stereocenters. Org. Lett. 2017, 19, 3231–3234; (g) Zhao, Q.-K.; Wu, X.; Yang, F.; Yan, P.-C.; Xie, J.-H.; Zhou, Q.-L. Catalytic Asymmetric Hydrogenation of 3-Ethoxycarbonyl Quinolin-2-ones and Coumarins. Org. Lett. 2021, 23, 3593–3598; (h) Zhu, Z.-H.; Ding, Y.-X.; Wu, B.; Zhou, Y.-G. Biomimetic Asymmetric Reduction of Tetrasubstituted Olefin 2,3-Disubstituted Inden-1-ones with Chiral and Regenerable NAD(P)H Model CYNAM. Org. Lett. 2021, 23, 7166–7170; (i) Yin, C.; Pan, Y.; Zhang, X.; Yin, Q. Catalytic Asymmetric Hydro-genation of Tetrasubstituted Unsaturated Lactams: an Efficient Ap-proach to Enantioenriched 3,4-Disubstituted Piperidines. Org. Lett. 2022, 24, 675–680; (j) Molinaro, C.; Scott, J. P.; Shevlin, M.; Wise, C.; Ménard, A.; Gibb, A.; Junker, E. M.; Lieberman, D. Catalytic, Asymmetric, and Stereodivergent Synthesis of Non-Symmetric β,β-Diaryl-α-Amino Acids. J. Am. Chem. Soc. 2015, 137, 999–1006; (k) Zhang, J.-H.; Xu, H.; Tang, X.; Dang, Y.; Zhang, F.; Ma, J. Highly Enantio- and Diastereoselective Hydrogenation of Cyclic Tetrasubstituted β-Enamido Phosphorus Derivatives. Angew. Chem. Int. Ed. 2023, 62, e202305315; (l) Wan, F.; Wang, N.; Zhu, Y.; Tang, C. Y.; Claverie, J.; Tang, W. J. Enantioselective Hydrogenation of Cyclic Tetrasubstituted-Olefinic Dehydroamino Acid Derivatives. Chem. Commun. 2021, 57, 5546–5549; (m) Song, S.; Zhu, S.-F.; Li, Y.; Zhou, Q.-L. Iridium-Catalyzed Enantioselective Hydrogenation of α,β-Unsaturated Carboxylic Acids with Tetrasubstituted Olefins. Org. Lett. 2013, 15, 3722–3725; (n) Arena, G.; Barreca, G.; Carcone, L.; Cini, E.; Marras, G.; Nedden, H. G.; Rasparini, M.; Roseblade, S.; Russo, A.; Taddei, M.; Zanotti-Gerosa, A. Rhodium-Catalyzed Enantioselective Hydrogenation of (E)-Enol Acetate Acids. Adv. Synth. Catal. 2013, 355, 1449–1454; (o) Du, X.; Xiao, Y.; Yang, Y.; Duan, Y.-N.; Li, F.; Hu, Q.; Chung, L. W.; Chen, G.-Q.; Zhang, X. Enantioselective Hydrogenation of Tetrasubstituted α,β-Unsaturated Carboxylic Acids Enabled by Cobalt(II) Catalysis: Scope and Mechanistic Insights. Angew. Chem. Int. Ed. 2021, 60, 11384–11390; (p) Chen, G.-Q.; Huang, J.-M.; Lin, B.-J.; Shi, C.; Zhao, L.-Y.; Ma, B.-D.; Ding, X.-B.; Yin, Q.; Zhang, X. Highly Enantioselective Hydrogenation of Tetra- and Tri-substituted α,β-Unsaturated Carboxylic Acids with Oxaspiro Diphosphine Ligands. CCS Chem. 2020, 2, 468–477; (q) Zhang, M.; Cui, P.; Zhang, K.; Shi, Z.; Cheng, X.; Ji, X.; Song, H.; Ke, B.; Qin, Y. Asymmetric Hydrogenation of All-Carbon Tetrasubstituted α-Acylpyrazole-β-alkyl Cycloalkenes. Org. Chem. Front. 2023, 10, 5070–5075.
- 24Based on previous mechanistic studies on the asymmetric hydrogenation of cyclic tetrasubstituted β-enamido phosphorus derivatives by Ma and co-workers (ref. 23k), a probable model for rationalizing the selectivity of this case was proposed in the Supporting Information.
- 25 Molinaro, C.; Shultz, S.; Roy, A.; Lau, S.; Trinh, T.; Angelaud, R.; O’Shea, P.; Abele, S.; Cameron, M.; Corley, E. A Practical Synthesis of Renin Inhibitor MK-1597 (ACT-178882) via Catalytic Enantioselective Hydrogenation and Epimerization of Piperidine Intermediate. J. Org. Chem. 2011, 76, 1062–107.
- 26 Lubov, D. P.; Talsi, E. P.; Bryliakov, K. P. Methods for Selective Benzylic C-H Oxofunctionalization of Organic Compounds. Russ. Chem. Rev. 2020, 89, 587–628.
- 27(a) Salvador, J.; Melo, M.; Neves, A. Copper-catalysed allylic oxidation of Δ5-steroids by t-butyl hydroperoxide. Tetrahedron Lett. 1997, 38, 119–122; (b) Yu, J.-Q.; Corey, E. J. Diverse Pathways for the Pal-ladium(II)-Mediated Oxidation of Olefins by tert-Butylhydroperoxide. Org. Lett. 2002, 4, 2727–2730; (c) Yu, J.-Q.; Corey, E. J. A Mild, Catalytic, and Highly Selective Method for the Oxidation of α,β-Enones to 1,4-Enediones. J. Am. Chem. Soc. 2003, 125, 3232−3233; (d) Catino, A. J.; Forslund, R. E.; Doyle, M. P. Dirhodium(II) Caprolactamate: An Exceptional Catalyst for Allylic Oxidation. J. Am. Chem. Soc. 2004, 126, 13622–13623; (e) Nechab, M.; Einhorn, C.; Einhorn, J. New Aerobic Oxidation of Benzylic Compounds: Efficient Catalysis by N-Hydroxy-3,4,5,6-tetraphenylphthalimide (NHTPPI)/ CuCl under Mild Conditions and Low Catalyst Loading. Chem. Commun. 2004, 1500–1501; (f) Zhao, P.; Guo, Y.; Luan, X. Total Synthesis of Dalesconol A by Pd(0)/Norbornene-Catalyzed Three-Fold Domino Reaction and Pd(II)-Catalyzed Trihydroxylation. J. Am. Chem. Soc. 2021, 143, 21270–21274; (g) Wang, G.-W.; Cheng, M.-X.; Ma, R.-S.; Yang, S.-D. Cu-Catalyzed Selective Cascade sp3 C–H Bond Oxidative Functionalization towards Isoxazoline Derivatives. Chem. Commun. 2015, 51, 6308–6311; (h) Li, T.; Li, J.; Zhu, Z.; Pan, W.; Wu, S. Cobalt(ii)-catalyzed benzylic oxidations with potassium persulfate in TFA/TFAA. RSC Adv. 2019, 9, 20879−20883.
- 28(a) Pandey, G.; Laha, R.; Singh, D. Benzylic C(sp3)–H Functionalization for C– N and C–O Bond Formation via Visible Light Photoredox Catalysis. J. Org. Chem. 2016, 81, 7161–7171; (b) Zhu, X.; Liu, Y.; Liu, C.; Yang, H.; Fu, H. Light and oxygen-enabled sodium trifluoro-methanesulfinate-mediated selective oxidation of C–H bonds. Green Chem. 2020, 22, 4357–4363; (c) Xie, P.; Xue, C.; Shi, S.; Du, D. Visible- Light-Driven Selective Air–oxygenation of C–H Bond via CeCl3 Catalysis in Water. ChemSusChem 2021, 14, 2689–2693; (d) Zhao, P.; Guo, Y.; Luan, X. Total Synthesis of Dalesconol A by Pd(0)/Norbornene- Catalyzed Three-Fold Domino Reaction and Pd(II)-Catalyzed Trihydroxylation. J. Am. Chem. Soc. 2021, 143, 21270–21274; (e) Li, S.; Zhu, B.; Lee, R.; Qiao, B.; Jiang, Z. Visible Light-Induced Selective Aerobic Oxidative Transposition of Vinyl Halides Using a Tetrahalogenoferrate (III) Complex Catalyst. Org. Chem. Front. 2018, 5, 380–385; (f) Laudadio, G.; Govaerts, S.; Wang, Y.; Ravelli, D.; Koolman, H. F. Fagnoni, M.; Djuric, S. W.; Noël, T. Angew. Chem. Int. Ed. 2018, 57, 4078−4082; (g) Shang, T.-Y.; Lu, L.-H.; Cao, Z.; Liu, Y.; He, W.-M.; Yu, B. Recent Advances of 1,2,3,5-Tetrakis(carbazol-9-yl)-4,6-dicyanobenzene (4CzlPN) in Photocatalytic Transformations. Chem. Commun. 2019, 55, 5408–5419; (h) Hendy, C. M.; Smith, G. C.; Xu, Z.; Lian, T.; Jui, N. T. Radical Chain Reduction via Carbon Dioxide Radical Anion (CO2•–). J. Am. Chem. Soc. 2021, 143, 8987–8992; (i) Thiruvengetam, P.; Chand, D. K. Controlled and predictably selective oxidation of activated and unactivated C(sp3)-H bonds catalyzed by a molybdenum-based metallomicellar catalyst in water. J. Org. Chem. 2022, 87, 4061−4077; (j) Qi, R.; Bai, T.; Tang, S.; Hou, M.; Zhang, Z.; Xie, W.; Deng, Y.; Zhou, H.; Qiu, G. Solvent-promoted photochemical carbonylation of benzylic C–H bonds under iron catalysis. Org. Biomol. Chem. 2023, 21, 5382−5386.
- 29(a) Du, W.; Xu, Y.; Wang, Y. Photoinduced Degradation of Orange II on Different Iron (Hydr)Oxides in Aqueous Suspension: Rate Enhancement on Addition of Hydrogen Peroxide, Silver Nitrate, and Sodium Fluoride. Langmuir 2008, 24, 175–181; (b) Hou, L.; Zhang, H.; Wang, L.; Chen, L. Ultrasound-Enhanced Magnetite Catalytic Ozonation of Tetracycline in Water. Chem. Eng. J. 2013, 229, 577–584.




