TAG-Assisted Liquid-Phase Synthesis and Structure Activity Relationship of Macolacin-Based Side-to-Tail Cyclopeptides Antibiotic
Haidi Li
Shanxi Provincial Key Laboratory of Drug Synthesis and Novel Pharmaceutical Preparation Technology, School of Pharmacy, Shanxi Medical University, Taiyuan, Shanxi, 030001 China
Medicinal Basic Research Innovation Center of Chronic Kidney Disease, Ministry of Education, Shanxi Medical University, Taiyuan, Shanxi, 030001 China
These authors contributed equally to this work.
Search for more papers by this authorYuankui Jin
Shanxi Provincial Key Laboratory of Drug Synthesis and Novel Pharmaceutical Preparation Technology, School of Pharmacy, Shanxi Medical University, Taiyuan, Shanxi, 030001 China
Medicinal Basic Research Innovation Center of Chronic Kidney Disease, Ministry of Education, Shanxi Medical University, Taiyuan, Shanxi, 030001 China
These authors contributed equally to this work.
Search for more papers by this authorMinfan Pei
Shanxi Provincial Key Laboratory of Drug Synthesis and Novel Pharmaceutical Preparation Technology, School of Pharmacy, Shanxi Medical University, Taiyuan, Shanxi, 030001 China
Medicinal Basic Research Innovation Center of Chronic Kidney Disease, Ministry of Education, Shanxi Medical University, Taiyuan, Shanxi, 030001 China
Search for more papers by this authorLinyan Zhang
Shanxi Provincial Key Laboratory of Drug Synthesis and Novel Pharmaceutical Preparation Technology, School of Pharmacy, Shanxi Medical University, Taiyuan, Shanxi, 030001 China
Medicinal Basic Research Innovation Center of Chronic Kidney Disease, Ministry of Education, Shanxi Medical University, Taiyuan, Shanxi, 030001 China
Search for more papers by this authorLianjun Wang
Shanxi Provincial Key Laboratory of Drug Synthesis and Novel Pharmaceutical Preparation Technology, School of Pharmacy, Shanxi Medical University, Taiyuan, Shanxi, 030001 China
Medicinal Basic Research Innovation Center of Chronic Kidney Disease, Ministry of Education, Shanxi Medical University, Taiyuan, Shanxi, 030001 China
Search for more papers by this authorYuxin Yang
Shanxi Provincial Key Laboratory of Drug Synthesis and Novel Pharmaceutical Preparation Technology, School of Pharmacy, Shanxi Medical University, Taiyuan, Shanxi, 030001 China
Medicinal Basic Research Innovation Center of Chronic Kidney Disease, Ministry of Education, Shanxi Medical University, Taiyuan, Shanxi, 030001 China
Search for more papers by this authorPeng Xiang
Shanxi Provincial Key Laboratory of Drug Synthesis and Novel Pharmaceutical Preparation Technology, School of Pharmacy, Shanxi Medical University, Taiyuan, Shanxi, 030001 China
Medicinal Basic Research Innovation Center of Chronic Kidney Disease, Ministry of Education, Shanxi Medical University, Taiyuan, Shanxi, 030001 China
Search for more papers by this authorCorresponding Author
Taigang Liang
Shanxi Provincial Key Laboratory of Drug Synthesis and Novel Pharmaceutical Preparation Technology, School of Pharmacy, Shanxi Medical University, Taiyuan, Shanxi, 030001 China
Medicinal Basic Research Innovation Center of Chronic Kidney Disease, Ministry of Education, Shanxi Medical University, Taiyuan, Shanxi, 030001 China
E-mail: [email protected]Search for more papers by this authorHaidi Li
Shanxi Provincial Key Laboratory of Drug Synthesis and Novel Pharmaceutical Preparation Technology, School of Pharmacy, Shanxi Medical University, Taiyuan, Shanxi, 030001 China
Medicinal Basic Research Innovation Center of Chronic Kidney Disease, Ministry of Education, Shanxi Medical University, Taiyuan, Shanxi, 030001 China
These authors contributed equally to this work.
Search for more papers by this authorYuankui Jin
Shanxi Provincial Key Laboratory of Drug Synthesis and Novel Pharmaceutical Preparation Technology, School of Pharmacy, Shanxi Medical University, Taiyuan, Shanxi, 030001 China
Medicinal Basic Research Innovation Center of Chronic Kidney Disease, Ministry of Education, Shanxi Medical University, Taiyuan, Shanxi, 030001 China
These authors contributed equally to this work.
Search for more papers by this authorMinfan Pei
Shanxi Provincial Key Laboratory of Drug Synthesis and Novel Pharmaceutical Preparation Technology, School of Pharmacy, Shanxi Medical University, Taiyuan, Shanxi, 030001 China
Medicinal Basic Research Innovation Center of Chronic Kidney Disease, Ministry of Education, Shanxi Medical University, Taiyuan, Shanxi, 030001 China
Search for more papers by this authorLinyan Zhang
Shanxi Provincial Key Laboratory of Drug Synthesis and Novel Pharmaceutical Preparation Technology, School of Pharmacy, Shanxi Medical University, Taiyuan, Shanxi, 030001 China
Medicinal Basic Research Innovation Center of Chronic Kidney Disease, Ministry of Education, Shanxi Medical University, Taiyuan, Shanxi, 030001 China
Search for more papers by this authorLianjun Wang
Shanxi Provincial Key Laboratory of Drug Synthesis and Novel Pharmaceutical Preparation Technology, School of Pharmacy, Shanxi Medical University, Taiyuan, Shanxi, 030001 China
Medicinal Basic Research Innovation Center of Chronic Kidney Disease, Ministry of Education, Shanxi Medical University, Taiyuan, Shanxi, 030001 China
Search for more papers by this authorYuxin Yang
Shanxi Provincial Key Laboratory of Drug Synthesis and Novel Pharmaceutical Preparation Technology, School of Pharmacy, Shanxi Medical University, Taiyuan, Shanxi, 030001 China
Medicinal Basic Research Innovation Center of Chronic Kidney Disease, Ministry of Education, Shanxi Medical University, Taiyuan, Shanxi, 030001 China
Search for more papers by this authorPeng Xiang
Shanxi Provincial Key Laboratory of Drug Synthesis and Novel Pharmaceutical Preparation Technology, School of Pharmacy, Shanxi Medical University, Taiyuan, Shanxi, 030001 China
Medicinal Basic Research Innovation Center of Chronic Kidney Disease, Ministry of Education, Shanxi Medical University, Taiyuan, Shanxi, 030001 China
Search for more papers by this authorCorresponding Author
Taigang Liang
Shanxi Provincial Key Laboratory of Drug Synthesis and Novel Pharmaceutical Preparation Technology, School of Pharmacy, Shanxi Medical University, Taiyuan, Shanxi, 030001 China
Medicinal Basic Research Innovation Center of Chronic Kidney Disease, Ministry of Education, Shanxi Medical University, Taiyuan, Shanxi, 030001 China
E-mail: [email protected]Search for more papers by this authorComprehensive Summary
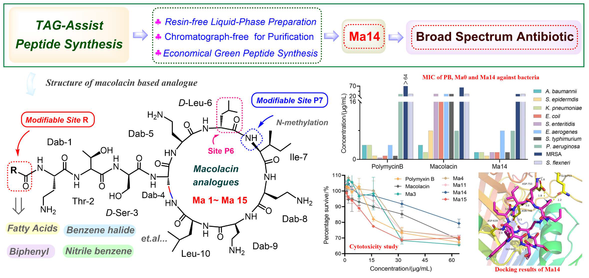
TAG-assisted peptide synthesis technology enables optimal conservation of Fmoc amino acid raw materials and chemical solvents while eliminating the need for intricate chromatographic purification processes. This work presents a 4,4'-diphenylphosphonoxy diphenylcarbinol tag-mediated liquid-phase synthesis approach for preparing side-to-tail cyclopeptides macolacin which has strong activity against gram-negative bacteria, and its 15 analogues containing four N-methylation modified cyclopeptides, as well as an investigation of their structure-activity relationship (SAR). The synthesis of macolacin analogues primarily focuses on the modifications of the N-methylation group of Ile-7 and the tail fatty acyl chain of macolacin. The incorporation of N-methylation for Ile-7, along with the dihalogenated or monohalogenated benzoic acids for tail modification, exhibited remarkable antibacterial efficacy and minimal hepatocellular toxicity in vitro. The present study identified an N-methylation-modified antimicrobial cyclopeptide Ma14 that exhibits rapid bactericidal efficacy against A. baumanii, etc., while showing reduced hepatocellular toxicity. Molecular docking simulations were conducted to investigate the binding of cyclopeptides to the outer membrane protein BamA of A. baumannii. The findings demonstrated the stable binding interactions of the cyclopeptides with the BamA protein and then presented a novel approach to explain the bacteriostatic mechanism of macolacin-based cyclopeptide antibiotics.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202400516-sup-0001-supinfo.pdfPDF document, 7.9 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Walia, K.; Mendelson, M.; Kang, G.; Venkatasubramanian, R.; Sinha, R.; Vijay, S.; Veeraraghavan, B.; Basnyat, B.; Rodrigues, C.; Bansal, N.; Ray, P.; Mathur, P.; Gopalakrishnan, R.; Ohri, V. C. How can lessons from the COVID-19 pandemic enhance antimicrobial resistance surveillance and stewardship. Lancet Infect Dis. 2023, 23, e301–e309.
- 2 Tarnawska, P.; Walczak, M.; Burkowska-But, A. Cemeteries and graveyards as potential reservoirs of antibiotic resistance genes and bacteria: a review. Environ. Chem. Lett. 2024, 22, 297–319.
- 3 Hatfull, G. F.; Dedrick, R. M.; Schooley, R. T. Phage therapy for antibiotic-resistant bacterial infections. Annu. Rev. Med. 2022, 73, 197–211.
- 4 Luther, A.; Urfer, M.; Zahn, M.; Müller, M.; Wang, S.-Y.; Mondal, M.; Vitale, A.; Hartmann, J.-B.; Sharpe, T.; Lo Monte, F.; et al. Chimeric peptidomimetic antibiotics against gram-negative bacteria. Nature 2019, 576, 452–458.
- 5 Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D. L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; Ouellette, M.; Outterson, K.; Patel, J.; Cavaleri, M., Cox, E. M.; Houchens, C. R.; Grayson, M. L.; Hansen, P.; Singh, N.; Theuretzbacher, U.; Magrini, N. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and developmentof new antibiotics. Lancet 2018, 18, 318–327.
- 6 Mahlapuu, M.; Björn, C.; Ekblom, J. Antimicrobial peptides as therapeutic agents: opportunities and challenges, critical reviews in biotechnology. Crit. Rev. Biotechnol. 2020, 40, 978–992.
- 7 Lakemeyer, M.; Zhao, W.; Mandl, F. A.; Hammann, P.; Sieber, S. A. Thinking outside the box-novel antibacterials to tackle the resistance crisis. Angew. Chem. Int. Ed. 2018, 57, 14440–14475.
- 8 Ma, P.; Chen, Y.; Shao, N.; Zhang, J.; Wu, Y.; Liu, R. Synergistic combination of biodegradable peptide polymer and curcumin as promising antibiotic substitution in aquaculture to alleviate the global challenge of antimicrobial resistance. Chin. J. Chem. 2022, 40, 2947–2955.
- 9 Yeaman, M. R.; Yount, N. Y. Mechanisms of antimicrobial peptide action and resistance. Pharmacol. Rev. 2003, 55, 27–55.
- 10 Zhang, Q. Y.; Yan, Z. B.; Meng, Y. M.; Hong, X. Y.; Shao, G.; Ma, J. J.; Cheng, X. R.; Liu, J.; Kang, J.; Fu, C. Y. Antimicrobial peptides: mechanism of action, activity and clinical potential. Military Med. Res. 2021, 8, 48.
- 11 Lazzaro, B. P.; Zasloff, M.; Rolff, J. Antimicrobial peptides: application informed by evolution. Science 2020, 368, 487.
- 12 Fantner, G. E.; Barbero, R. J.; Gray, D. S.; Belcher, A. M. Kinetics of antimicrobial peptide activity measured on individual bacterial cells using high-speed atomic force microscopy. Nat. Nanotechnol. 2010, 5, 280–285.
- 13 Hansen, I. K. Ø.; Lövdahl, T.; Simonovic, D.; Hansen, K. Ø.; Andersen, A. J. C.; Devold, H.; Richard, C. S. M.; Andersen, J. H.; Strøm, M. B.; Haug, T. Antimicrobial activity of small synthetic peptides based on the marine peptide turgencin A: prediction of antimicrobial peptide sequences in a natural peptide and strategy for optimization of potency. Int. J. Mol. Sci. 2020, 21, 5460.
- 14 Hancock, R. E. W.; Alford, M. A.; Haney, E. F. Antibiofilm activity of host defence peptides: complexity provides opportunities. Nat. Rev. Microbiol. 2021, 19, 786–797.
- 15 Mookherjee, N.; Anderson, M. A.; Haagsman, H. P.; Davidson, D. J. Antimicrobial host defence peptides: functions and clinical potential. Nat. Rev.Drug. Discov. 2020, 19, 311–332.
- 16 Ha Gan, B.; Gaynord, J.; Rowe, S. M.; Deingruber, S. T.; Spring, D. R. The multifaceted nature of antimicrobial peptides: current synthetic chemistry approaches and future directions. Chem. Soc. Rev. 2021, 50, 7820–7880.
- 17 Wang, L.; Wang, N.; Zhang, W.; Cheng, X.; Yan, Z.; Shao, G.; Wang, X.; Wang, R.; Fu, C. Therapeutic peptides: current applications and future directions. Sig. Transduct. Target Ther. 2022, 7, 1–27.
- 18 Jin, X.; Park, O. J.; Hong, S. H. Incorporation of non-standard amino acids into proteins: challenges, recent achievements, and emerging applications, Appl. Microbiol. Biotechnol. 2019, 103, 2947–2958.
- 19 Sun, Y.; Tang, W.; Wang, M.; Ni, H.; Long, Y. Q. Synthesis of piperazic acid-containing cyclodepsipeptide core of verucopeptin. Chin. J. Chem. 2023, 41, 2077–2081.
- 20 Chen, X.; Li, B.; Tong, H.; Qi, L.; He, G.; Chen, G. Palladium-catalyzed methionine-facilitated β and γC(sp3)-H arylation of N-terminal aliphatic amino acids of peptides. Chin. J. Chem. 2022, 40, 2502–2506.
- 21
Ferrazzano, L.; Catani, M.; Cavazzini, A.; Martelli, G.; Corbisiero, D.; Cantelmi, P.; Fantoni, T.; Mattellone, A.; Luca, C. D.; Felletti, S.; Cabri, W.; Tolomelli, A. Sustainability in peptide chemistry: Current synthesis and purification technologies and future challenges. Green Chem. 2022, 94, 975–1020.
10.1039/D1GC04387K Google Scholar
- 22 Martin, V.; Egelund, P. H. G.; Johansson, H.; Quement, S. T. L.; Wojcik, F.; Pedersen, D. S. Greening the synthesis of peptide therapeutics: an industrial perspective. RSC. Adv. 2020, 10, 42457–42492.
- 23 Sharma, A.; Kumar, A.; de la Torre, B. G.; Albericio, F. Liquid-phase peptide synthesis (LPPS): A third wave for the preparation of peptides. Chem. Rev. 2022, 122, 13516–13546.
- 24 Wang, Z.; Koirala, B.; Hernandez, Y.; Zimmerman, M.; Park, S.; Perlin, D. S.; Brady, S. F. A naturally inspired antibiotic to target multidrug- resistant pathogens. Nature 2022, 601, 606.
- 25 Patil, N. A.; Ma, W.; Jiang, X.; He, X.; Yu, H. H.; Wickremasinghe, H.; Wang, J.; Thompson, P. E.; Velkov, T.; Roberts, K. D.; Li, J. Critical role of position 10 residue in the polymyxin antimicrobial activity. J. Med. Chem. 2023, 66, 2865–2876.
- 26 Li, J.; Guan, D.; Chen, F.; Shi, W.; Lan, L.; Huang, W. Total and semisyntheses of polymyxin analogues with 2-Thr or 10-Thr modifications to decipher the structure-activity relationship and improve the antibacterial activity. J. Med. Chem. 2021, 64, 5746–5765.
- 27 Gallardo-Godoy, A.; Muldoon, C.; Becker, B.; Elliott, A. G.; Lash, L. H.; Huang, J. X.; Butler, M. S.; Pelingon, R.; Kavanagh, A. M.; Ramu, S.; Phetsang, W.; Blaskovich, M. A. T.; Cooper, M. A. Activity and predicted nephrotoxicity of synthetic antibiotics based on polymyxin B. J. Med. Chem. 2016, 59, 1068–1077.
- 28 Li, H. D.; Jin, Y. K.; Wang, L. J.; Zhang, L. Y.; Liu, T. Y.; Liu, Y.; Liang, T. G. One-pot and sustainable liquid-Phase peptide extension for synthesis of C-terminal amidation peptides aided by small molecular tags. Org. Chem. Front. 2023, 10, 6158–6165.
- 29 Li, H. D.; Li, J. Y.; Chao, J.; Zhang, Z. X.; Qin, C. G. Head-to-tail cyclization for the synthesis of naturally occurring cyclic peptides on organophosphorus small-molecular supports. Org. Chem. Front. 2022, 9, 946–952.
- 30 Li, H. D.; Chao, J.; Tian, G.; Hasan, J.; Jin, Y. T.; Zhang, Z. X.; Qin, C. G. Resin-free peptide synthesis mediated by tri(4-benzoylphenyl) phosphate (TBP) derivatives as small-molecule supports. Org. Chem. Front. 2020, 7, 689–696.
- 31 Li, H. D.; Chao, J.; Zhang, Z. X.; Tian, G.; Li, J.; Chang, N. H.; Qin, C. G. Liquid-phase total synthesis of plecanatide aided by diphenyl phosphinyloxyl diphenyl ketone (DDK) derivatives. Org. Lett. 2020, 22, 3323–3328.
- 32 Li, H. D.; Chao, J.; Tian, G.; Hasan, J.; Jin, Y. T.; Zhang, Z. X.; Qin, C. G. Synthesis of tri (4-formylphenyl) phosphonate (TFP) derivatives as recyclable triple-equivalent supports of peptide-synthesis. J. Org. Chem. 2020, 85, 6271–6280.
- 33 Li, H. D.; Ren, J.; Li, J. Y.; Zhang, Z. X.; Chang, N. H.; Qin, C. G. Greener liquid-phase synthesis and the ACE inhibitory structure-activity relationship of an anti-SARS octapeptide. Org. Biomol. Chem. 2020, 18, 8433–8442.
- 34 Chatterjee, J.; Gilon, C.; Hoffman, A.; Kessler, H. N-Methylation of peptides: A new perspective in medicinal chemistry. Acc. Chem. Res. 2008, 41, 1331–1342.
- 35 Song, H.; Burton, A. J.; Shirran, S. L.; Fahrig-Kamarauskaitė, J.; Kaspar, H.; Muir, T. W.; Künzler, M.; Naismith, J. H. Engineering of a peptide α-N-methyltransferase to methylate non-proteinogenic amino acid. Angew. Chem. Int. Ed. 2021, 60, 14319–14323.
- 36 Hosono, Y.; Uchida, S.; Shinkai, M.; Townsend, C. E.; Kelly, C. N.; Naylor, M. R.; Lee, H.-W.; Kanamitsu, K.; Ishii, M.; Ueki, R.; Ueda, T.; Takeuchi, K.; Sugita, M.; Akiyama, Y.; Lokey, S. R.; Morimoto, J.; Sando, S. Amide-to-ester substitution as a stable alternative to N-methylation for Increasing membrane permeability in cyclic peptides. Nat Commun. 2023, 14, 1416.
- 37 Biron, E.; Chatterjee, J.; Ovadia, O.; Langenegger, D.; Brueggen, J.; Hoyer, D.; Schmid, H. A.; Jelinek, R.; Gilon, C.; Hoffman, A.; Kessler, H. Improving oral bioavailability of peptides by multiple N-methylation: somatostatin analogues. Angew. Chem. Int. Ed. 2008, 47, 2595–2599.
- 38 Chatterjee, J.; Rechenmacher, F.; Kessler, H. N-Methylation of peptides and proteins: an important element for modulating biological functions. Angew. Chem. Int. Ed. 2013, 52, 254–269.
- 39 Mas-Moruno, C.; Beck, J. G.; Doedens, L.; Frank, A. O.; Marinelli, L.; Cosconati, S.; Novellino, E.; Kessler, H. Increasing αvβ3 Selectivity of the anti-angiogenic drug cilengitide by N-methylation. Angew. Chem. Int. Ed. 2011, 50, 9496–9500.
- 40 Chatterjee, J.; Ovadia, O.; Zahn, G.; Marinelli, L.; Hoffman, A.; Gilon, C.; Kessler, H. Multiple N-methylation by a designed approach enhances receptor selectivity. J. Med. Chem. 2007, 50, 5878–5881.
- 41 Dechantsreiter, M. A.; Planker, E.; Mathä, B.; Lohof, E.; Hölzemann, G.; Jonczyk, A.; Goodman, S. L.; Kessler, H. N-Methylated cyclic RGD peptides as highly active and selective αVβ3 integrin antagonists. J. Med. Chem. 1999, 42, 3033–3040.
- 42 Tsuji, B. T.; Pogue, J. M.; Zavascki, A. P.; Paul, M.; Daikos, G. L.; Forrest, A.; Giacobbe, D. R.; Viscoli, C.; Giamarellou, H.; Karaiskos, I.; Kaye, D.; Mouton, J. W.; Tam, V. H.; Thamlikitkul, V.; Wunderink, R. G.; Li, J.; Nation, R. L.; Kaye, K. S. International consensus guidelines for the optimal use of the polymyxins: endorsed by the American College of Clinical Pharmacy (ACCP), European Society of Clinical Microbiology and Infectious Diseases (ESCMID), Infectious Diseases Society of America (IDSA), International Society for Anti-Infective Pharmacology (ISAP), Society of Critical Care Medicine (SCCM), and Society of Infectious Diseases Pharmacists (SIDP). Pharmacotherapy: J. Hum. Pharm. Drug Therapy. 2019, 39, 10–39.
- 43 Yu, H. Y.; Tu, C. H.; Yip, B. S.; Chen, H. L.; Cheng, H. T.; Huang, K. C.; Lo, H. J.; Cheng, J. W. Easy strategy to increase salt resistance of antimicrobial peptides. Antimicrob. Agents Chemother. 2011, 55, 4918–4921.
- 44 Pankey, G. A.; Sabath, L. D. Clinical relevance of bacteriostatic versus bactericidal mechanisms of action in the treatment of gram-positive bacterial infections. Clin. Infect. Dis. 2004, 38, 864–870.
- 45 Guo, Y.; Xun, M.; Han, J. A bovine myeloid antimicrobial peptide (BMAP-28) and its analogs kill pan-drug-resistant acinetobacter baumannii by interacting with outer membrane protein A (OmpA). Medicine 2018, 97, e12832.
- 46 Nie, D.; Hu, Y.; Chen, Z.; Li, M.; Hou, Z.; Luo, X.; Mao, X.; Xue, X. Outer membrane protein A (OmpA) as a potential therapeutic target for acinetobacter baumannii infection. J. Biomed. Sci. 2020, 27, 26.
- 47 Shen, C.; Chang, S.; Luo, Q.; Chan, K. C.; Zhang, Z.; Luo, B.; Xie, T.; Lu, G.; Zhu, X.; Wei, X.; Dong, C.; Zhou, R.; Zhang, X.; Tang, X.; Dong, H. Structural basis of BAM-mediated outer membrane β-barrel protein assembly. Nature 2023, 617, 185–193.
- 48 Robinson, J. A. Folded synthetic peptides and other molecules targeting outer membrane protein complexes in gram-negative bacteria. Front. Chem. 2019, 7, 45.




