In Situ Liquid Cell Transmission Electron Microscopy Observations of Growth and Anticorrosion Behaviors of AuCl3 Shell on Au Nanobipyramids
Wei Wei
School of Information Technology, Jiangsu Open University, Nanjing, Jiangsu, 210036 China
SEU-FEI Nano-Pico Center, Key Laboratory of MEMS of Ministry of Education, Collaborative Innovation Center for Micro/Nano Fabrication, Device and System, Southeast University, Nanjing, Jiangsu, 210096 China
Search for more papers by this authorJun Sun
School of Information Technology, Jiangsu Open University, Nanjing, Jiangsu, 210036 China
Search for more papers by this authorSongtao Zhang
Testing Center, Yangzhou University, Yangzhou, Jiangsu, 225009 China
Search for more papers by this authorCorresponding Author
Litao Sun
SEU-FEI Nano-Pico Center, Key Laboratory of MEMS of Ministry of Education, Collaborative Innovation Center for Micro/Nano Fabrication, Device and System, Southeast University, Nanjing, Jiangsu, 210096 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Feng Xu
SEU-FEI Nano-Pico Center, Key Laboratory of MEMS of Ministry of Education, Collaborative Innovation Center for Micro/Nano Fabrication, Device and System, Southeast University, Nanjing, Jiangsu, 210096 China
E-mail: [email protected]; [email protected]Search for more papers by this authorWei Wei
School of Information Technology, Jiangsu Open University, Nanjing, Jiangsu, 210036 China
SEU-FEI Nano-Pico Center, Key Laboratory of MEMS of Ministry of Education, Collaborative Innovation Center for Micro/Nano Fabrication, Device and System, Southeast University, Nanjing, Jiangsu, 210096 China
Search for more papers by this authorJun Sun
School of Information Technology, Jiangsu Open University, Nanjing, Jiangsu, 210036 China
Search for more papers by this authorSongtao Zhang
Testing Center, Yangzhou University, Yangzhou, Jiangsu, 225009 China
Search for more papers by this authorCorresponding Author
Litao Sun
SEU-FEI Nano-Pico Center, Key Laboratory of MEMS of Ministry of Education, Collaborative Innovation Center for Micro/Nano Fabrication, Device and System, Southeast University, Nanjing, Jiangsu, 210096 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Feng Xu
SEU-FEI Nano-Pico Center, Key Laboratory of MEMS of Ministry of Education, Collaborative Innovation Center for Micro/Nano Fabrication, Device and System, Southeast University, Nanjing, Jiangsu, 210096 China
E-mail: [email protected]; [email protected]Search for more papers by this authorComprehensive Summary
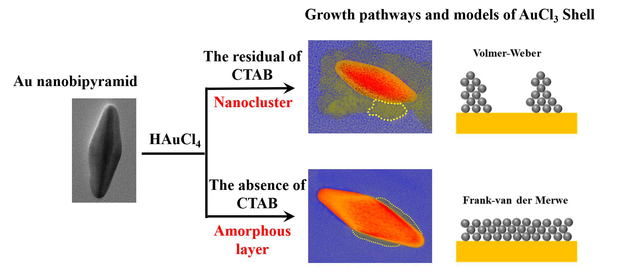
Designing new materials and architectures to maintain activity and stability requires a better understanding on the anticorrosion dynamics of nanoparticles. Under-coordinated atoms on the surface of nanoparticles can be protected by deposited shells. Real-time observation on how protective shells grow and play a role is challenging but worthwhile. Here, protective effects of AuCl3 shells on Au nanobipyramids (NBPs) are studied in HAuCl4 aqueous solutions by in-situ liquid cell transmission electron microscopy (LCTEM). This study is the first to observe the formation of Au-AuCl3 core-shell nanostructure and the corresponding anticorrosion behaviors of AuCl3 deposited shell. The presence of CTAB can substantially influence the growth mode and structure of AuCl3 shell, by a direct or indirect way, intervene the dissolution of Au NBP. These growth or dissolution kinetics here revealed at the nanoscale provide insights towards engineering of the surface anticorrosion to pursue Au nanoparticles with improved stability in acidic environment.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202400503-sup-0001-supinfo.pdfPDF document, 2 MB |
Appendix S1: Supporting Information |
| cjoc202400503-sup-0002-supinfo.mp4MPEG-4 video, 13.9 MB |
Appendix S2: Supporting Information |
| cjoc202400503-sup-0003-supinfo.mp4MPEG-4 video, 12.9 MB |
Appendix S3: Supporting Information |
| cjoc202400503-sup-0004-supinfo.mp4MPEG-4 video, 8.1 MB |
Appendix S4: Supporting Information |
| cjoc202400503-sup-0005-supinfo.mp4MPEG-4 video, 9.9 MB |
Appendix S5: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Daniel, M. C.; Astruc, D. Gold Nanoparticles: Assembly, Supramolecular Chemistry, Quantum-Size-Related Properties, and Applications Toward Biology, Catalysis, and Nanotechnology. Chem. Rev. 2004, 104, 293–346.
- 2 Saha, K. Gold Nanoparticles in Chemical and Biological Sensing. Chem. Rev. 2011, 112, 2739–2779.
- 3
Astruc, D.; Feng, L.; Aranzaes, J. R. Nanoparticles as Recyclable Catalysts: The Frontier between Homogeneous and Heterogeneous Catalysis. Angew. Chem. Int. Ed. 2010, 44, 7852–7872.
10.1002/anie.200500766 Google Scholar
- 4 Gorin, D. J.; Sherry, B.; Toste, F. D. Ligand Effects in Homogeneous Au Catalysis. Chem. Rev. 2008, 108, 3351–3378.
- 5 Luo, W. J.; Zhu, C. F.; Su, S.; Li, D.; He, Y.; Huang, Q.; Fan, C. Self-Catalyzed, Self-Limiting Growth of Glucose Oxidase-Mimicking Gold Nanoparticles. ACS Nano 2010, 4, 7451–7458.
- 6 Yin, H. J.; Tang, H. J.; Wang, D.; Gao, Y.; Tang, Z. Facile Synthesis of Surfactant-Free Au Cluster/Graphene Hybrids for High-Performance Oxygen Reduction Reaction. ACS Nano 2012, 6, 8288–8297.
- 7 Shan, H.; Gao, W. P.; Xiong, Y. L.; Shi, F.; Yan, Y.; Ma, Y.; Shang, W.; Tao, P.; Song, C.; Deng, T.; Zhang, H.; Yang, D.; Pan, X.; Wu, J. Nanoscale Kinetics of Asymmetrical Corrosion in Core-Shell Nanoparticles. Nat. Commun. 2018, 9, 1011–1018.
- 8 Tao, A. R.; Habas, S.; Yang, P. Shape Control of Colloidal Metal Nanocrystals. Small 2008, 4, 310–325.
- 9 Zhang, J.; Sasaki, K.; Sutter, E.; Adzic, R. R. Stabilization of Platinum Oxygen-Reduction Electrocatalysts Using Gold Clusters. Science 2007, 315, 220–222.
- 10 Sasaki, K.; Naohara, H.; Choi, Y. M.; Cai, Y.; Chen, W. F.; Liu, P.; Adzic, R. R. Highly Stable Pt Monolayer on PdAu Nanoparticle Electrocatalysts for the Oxygen Reduction Reaction. Nat. Commun. 2011, 3, 1115–1119.
- 11 Cai, Y.; Ma, C.; Zhu, Y. M.; Wang, J. X.; Adzic, R. R. Low-Coordination Sites in Oxygen-Reduction Electrocatalysis: Their Roles and Methods for Removal. Langmuir 2011, 27, 8540–8547.
- 12 Wang, C.; Chi, M.; Li, D.; Strmcnik, D.; Vliet, D.; Wang, G.; Komanicky, V.; Chang, K. C.; Paulikas, A. P.; Tripkovic, D.; Pearson, J.; More, K. L.; Markovic, N. M.; Stamenkovic, V. R. Design and Synthesis of Bimetallic Electrocatalyst with Multilayered Pt-Skin Surfaces. J. Am. Chem. Soc. 2011, 133, 14396–14403.
- 13 Snyder, J.; McCue, I.; Livi, K.; Erlebacher, J. Structure/Processing/ Properties Relationships in Nanoporous Nanoparticles as Applied to Catalysis of the Cathodic Oxygen Reduction Reaction. J. Am. Chem. Soc. 2012, 134, 8633–8645.
- 14 Gilroy, K. D.; Ruditskiy, A.; Peng, H.; Qin, D.; Xia, Y. Bimetallic Nanocrystals: Syntheses, Properties, and Applications. Chem. Rev. 2016, 116, 10414–10472.
- 15 Wang, W.; Xu, T.; Chen, J.; Shangguan, J.; Dong, H.; Ma, H.; Zhang, Q.; Yang, J.; Bai, T.; Guo, Z.; Fang, H.; Zheng, H.; Sun, L. Solid-Liquid-Gas Reaction Accelerated by Gas Molecule Tunnelling-Like Effect. Nat. Mater. 2022, 21, 859–863.
- 16Jiang, Y. J; Dai, S. Exploration of Strong Metal-Support Interaction in Heterogeneous Catalysts by in Situ Transmission Electron Microscopy. Chin. J. Chem. 2024, 42, 1004–1008.
10.1002/cjoc.202300586 Google Scholar
- 17 Sun, Z. F.; Pan, J. H.; Chen, W. W.; Chen, H. Y.; Zhou, S. H.; Wu, X. Y.; Wang, Y. S.; Kim, K.; Li, J.; Liu, H. D.; Yuan, Y. F.; Wang, J. W.; Su, D.; Peng, D. L.; Zhang, Q. B. Electrochemical Processes and Reactions in Rechargeable Battery Materials Revealed via in Situ Transmission Electron Microscopy. Adv. Energy Mater. 2024, 14, 2303165.
- 18
Sun, Z. F.; Li, M.; Xiao, B. S.; Liu, X.; Lin, H. C.; Jiang, B.; Liu, H. D.; Li, M. C.; Peng, D. L.; Zhang, Q. B. In Situ Transmission Electron Microscopy for Understanding Materials and Interfaces Challenges in All-Solid- State Lithium Batteries. eTransportation 2022, 14, 100203.
10.1016/j.etran.2022.100203 Google Scholar
- 19 Zhang, J. Y.; Sun, Z. F.; Kang, Z. W.; Lin, H. C.; Liu, H. D.; He, Y.; Zeng, Z. Y.; Zhang, Q. B. Unveiling the Dynamic Oxidative Etching Mechanisms of Nanostructured Metals/Metallic Oxides in Liquid Media through in Situ Transmission Electron Microscopy. Adv. Funct. Mater. 2022, 32, 202204976.
- 20 Zhang, J. Y.; Xiao, B. S.; Zhao, J. H.; Li, M.; Lin, H. C.; Kang, Z. W.; Wu, X. W.; Liu, H. D.; Peng, D. L.; Zhang, Q. B. Understanding the Growth Mechanisms of Metal-Based Core-Shell Nanostructures Revealed by In Situ Liquid Cell Transmission Electron Microscopy. J. Energy Chem. 2022, 71, 370–383.
- 21 Dalmonico, G. M. L.; Ihiawakrim, D.; Ortiz, N.; Barreto, A. G.; Marcellos, C. F. C.; Farina, M.; Ersen, O.; Rossi, A. L. Live Visualization of the Nucleation and Growth of Needle-Like Hydroxyapatite Crystals in Solution by in Situ TEM. Cryst. Growth Des. 2022, 22, 4828–4837.
- 22 Nielsen, M. H.; Aloni, S.; Yoreo, J. J. D. In Situ TEM Imaging of CaCO3 Nucleation Reveals Coexistence of Direct and Indirect Pathways. Science 2014, 345, 1158–1162.
- 23 Qin, F.; Wang, Z.; Wang, Z. L. Anomalous Growth and Coalescence Dynamics of Hybrid Perovskite Nanoparticles Observed by Liquid-Cell Transmission Electron Microscopy. ACS Nano 2016, 10, 9787–9790.
- 24 Chen, X.; Li, C.; Kong, X.; Cao, H. L.; Wang, H. L.; Zhou, X. Q. Direct Observation of Growth and Self-Assembly of Pt Nanoclusters in Water with the Aid of a Triblock Polymer Using in Situ Liquid Cell Transmission Electron Microscopy. Chin. J. Chem. 2017, 35, 1278–1283.
- 25 Zeng, Z.; Liang, W.; Liao, H. G.; Xin, H. L.; Chu, Y. H.; Zheng, H. Visualization of Electrode-Electrolyte Interfaces in LiPF6/EC/DEC Electrolyte for Lithium Ion Batteries via in Situ TEM. Nano Lett. 2014, 14, 1745–1750.
- 26 Aleksandar, R.; Vereecken, P. M.; Hannon, J. B.; Searson, P. C.; Ross, F. M. Quantifying Electrochemical Nucleation and Growth of Nanoscale Clusters Using Real-Time Kinetic Data. Nano Lett. 2020, 6, 238–242.
- 27 Anand, U.; Lu, J.; Loh, D.; Aabdin, Z.; Mirsaidov, U. Hydration Layer- Mediated Pairwise Interaction of Nanoparticles. Nano Lett. 2016, 16, 786–790.
- 28 Tan, S. F.; Anand, U.; Mirsaidov, U. Interactions and Attachment Pathways between Functionalized Gold Nanorods. ACS Nano 2017, 11, 1633–1640.
- 29 Fu, X.; Chen, B.; Tang, J.; Hassan, M. T.; Zewail, A. H. Imaging Rotational Dynamics of Nanoparticles in Liquid by 4D Electron Microscopy. Science 2017, 355, 494–498.
- 30 Wei, W.; Zhang, H.; Wang, W.; Dong, M.; Nie, M.; Sun, L.; Xu, F. Observing the Growth of Pb3O4 Nanocrystals by in situ Liquid Cell Transmission Electron Microscopy. ACS Appl. Mater. Inter. 2019, 11, 24478–24484.
- 31 Dong, M.; Fu, R.; Min, H.; Zhang, Q.; Dong, H.; Pan, Y.; Sun, L.; Wei, W.; Qin, M.; Zhu, Z.; Xu, F. In Situ Liquid Cell Transmission Electron Microscopy Investigation on the Dissolution-Regrowth Mechanism Dominating the Shape Evolution of Silver Nanoplates. Cryst. Growth Des. 2021, 21, 1314–1322.
- 32 Jungjohann, K. L.; Bliznakov, S.; Sutter, P. W.; Stach, E. A.; Sutter, E. A. In Situ Liquid Cell Electron Microscopy of the Solution Growth of Au-Pd Core-Shell Nanostructures. Nano Lett. 2013, 13, 2964–2970.
- 33 Tan, S. F.; Chee, S. W.; Lin, G.; Bosman, M.; Lin, M.; Mirsaidov, U.; Nijhuis, C. A. Real-Time Imaging of the Formation of Au-Ag Core-Shell Nanoparticles. J. Am. Chem. Soc. 2016, 138, 5190–5193.
- 34 Chen, F. C.; Chen, J. Y.; Lin, Y. H.; Kuo, M. Y.; Hsu, Y. J.; Wu, W. W. In Situ TEM Observation of Au-Cu2O Core-Shell Growth in Liquids. Nanoscale 2019, 11, 10486–10492.
- 35 Schneider, N. M.; Norton, M. M.; Mendel, B. J.; Grogan, J. M.; Ross, F. M.; Bau, H. H. Electron-Water Interactions and Implications for Liquid Cell Electron Microscopy. J. Phys. Chem. C 2014, 118, 22373–22382.
- 36 Zhang, Z.; Lagally, M. G. Atomistic Processes in the Early Stages of Thin-Film Growth. Science 1997, 276, 377–383.
- 37 Lozoyoy, K. A.; Kokhanenko, A. P.; Dirko, V. V.; Akimenko, N. Y.; Voitsekhovskii, A. V. Evolution of Epitaxial Quantum Dots Formed by Volmer−Weber Growth Mechanism. Cryst. Growth Des. 2019, 19, 7015–7021.
- 38 Zhang, J.; Xue, D. J.; Zhan, X. J.; Li, Z.; Zeng, D. W.; Song, H. B. Versatile Solution-Processed Synthesis of Two-Dimensional Ultrathin Metal Chalcogenides Following Frank-van der Merwe Growth. ACS Appl. Mater. Interfaces 2017, 9, 27102–27110.
- 39 Alpay, D.; Peng, L.; Marks, L. D. Are Nanoparticle Corners Round? J. Phys. Chem. C 2015, 119, 21018–21023.
- 40 Marks, L. D.; Peng, L. Nanoparticle Shape, Thermodynamics and Kinetics. J. Phys. Condens. Matter 2016, 28, 053001–053005.
- 41 Nie, Z.; Fava, D.; Kumacheva, E.; Zou, S.; Walker, G. C.; Rubinstein, M. Self-Assembly of Metal-Polymer Analogues of Amphiphilic Triblock Copolymers. Nat. Mater. 2007, 6, 609–614.
- 42 Wei, W.; Bai, T.; Fu, R.; Sun, L.; Wang, W.; Dong, M.; Chen, L.; Guo, Z.; Xu, F. Unravelling the Shell Growth Pathways of Au-Ag Core-Shell Nanoparticles by in Situ Liquid Cell Transmission Electron Microscopy. Nanoscale 2021, 13, 3136–3143.




