Electrolyte Effects in Electrocatalytic Kinetics†
Xiao-Yu Li
State Key Laboratory of Physical Chemistry of Solid Surfaces, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, Fujian, 361005 China
Search for more papers by this authorZhi-Ming Zhang
State Key Laboratory of Physical Chemistry of Solid Surfaces, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, Fujian, 361005 China
Search for more papers by this authorXin-Xin Zhuang
State Key Laboratory of Physical Chemistry of Solid Surfaces, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, Fujian, 361005 China
Search for more papers by this authorZe-Tong Jia
State Key Laboratory of Physical Chemistry of Solid Surfaces, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, Fujian, 361005 China
Search for more papers by this authorCorresponding Author
Tao Wang
State Key Laboratory of Physical Chemistry of Solid Surfaces, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, Fujian, 361005 China
Innovation Laboratory for Sciences and Technologies of Energy Materials of Fujian Province (IKKEM), Xiamen, Fujian, 361005 China
E-mail: [email protected]Search for more papers by this authorXiao-Yu Li
State Key Laboratory of Physical Chemistry of Solid Surfaces, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, Fujian, 361005 China
Search for more papers by this authorZhi-Ming Zhang
State Key Laboratory of Physical Chemistry of Solid Surfaces, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, Fujian, 361005 China
Search for more papers by this authorXin-Xin Zhuang
State Key Laboratory of Physical Chemistry of Solid Surfaces, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, Fujian, 361005 China
Search for more papers by this authorZe-Tong Jia
State Key Laboratory of Physical Chemistry of Solid Surfaces, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, Fujian, 361005 China
Search for more papers by this authorCorresponding Author
Tao Wang
State Key Laboratory of Physical Chemistry of Solid Surfaces, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, Fujian, 361005 China
Innovation Laboratory for Sciences and Technologies of Energy Materials of Fujian Province (IKKEM), Xiamen, Fujian, 361005 China
E-mail: [email protected]Search for more papers by this author† Dedicated to the Special Issue of Emerging Investigators in 2024.
Abstract
Comprehensive Summary
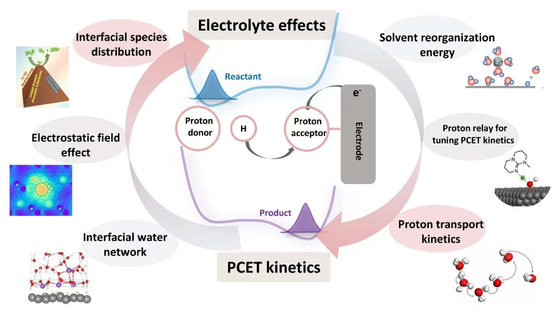
Tuning electrolyte properties is a widely recognized strategy to enhance activity and selectivity in electrocatalysis, drawing increasing attention in this domain. Despite extensive experimental and theoretical studies, debates persist about how various electrolyte components influence electrocatalytic reactions. We offer a concise review focusing on current discussions, especially the contentious roles of cations. This article further examines how different factors affect the interfacial solvent structure, particularly the hydrogen-bonding network, and delves into the microscopic kinetics of electron and proton-coupled electron transfer. We also discuss the overarching influence of solvents from a kinetic modeling perspective, aiming to develop a robust correlation between electrolyte structure and reactivity. Lastly, we summarize ongoing research challenges and suggest potential directions for future studies on electrolyte effects in electrocatalysis.
Key Scientists
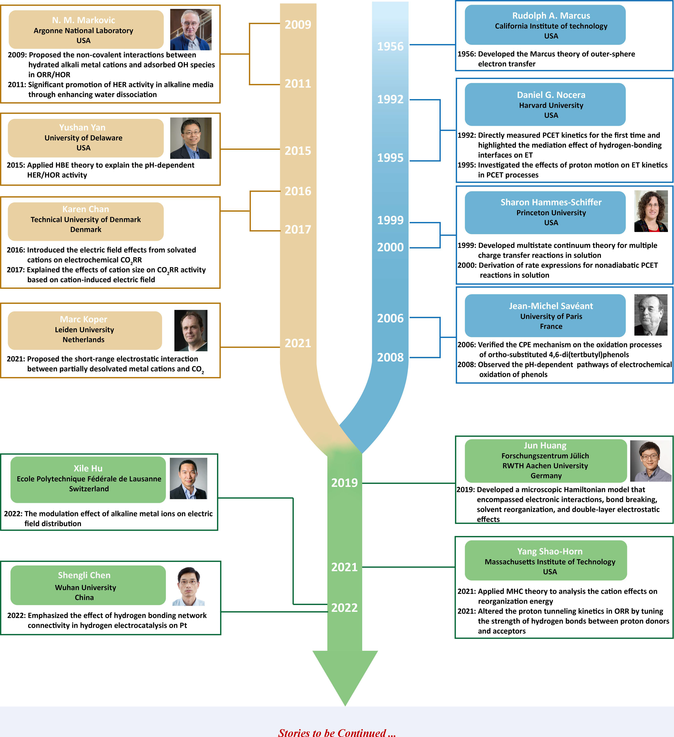
In 1956, Marcus theory was developed to describe the mechanism of outer-sphere electron transfer (OS-ET). In 1992, Nocera et al. directly measured proton-coupled electron transfer (PCET) kinetics for the first time, and their subsequent research in 1995 investigated the effects of proton motion on electron transfer (ET) kinetics. In 1999 and 2000, Hammes-schiffer et al. developed the multistate continuum theory for multiple charge reactions and deduced the rate expressions for nonadiabatic PCET reactions in solution, laying the theoretical foundation for the analysis of PCET kinetics in electrochemical processes. In 2006, Saveant et al. verified the concerted proton and electron transfer (CPET) mechanism in the oxidation of phenols coupled with intramolecular amine-driven proton transfer (PT). Their subsequent work in 2008 reported the pH-dependent pathways of electrochemical oxidation of phenols.
Electrolyte effects in electrocatalysis have gained emphasis in recent years. In 2009, Markovic's pioneering work proposed non-covalent interactions between hydrated alkaline cations and adsorbed OH species in oxygen reduction reaction (ORR)/hydrogen oxidation reaction (HOR). In 2011, Markovic et al. significantly enhanced hydrogen evolution reaction (HER) activity in alkaline solution by improving water dissociation, which was assumed to dominate the sluggish HER kinetics in such media. In comparation, Yan et al. applied hydrogen binding energy (HBE) theory in 2015 to explain the pH-dependent HER/HOR activity. Cations play a significant role in regulating the selectivity and activity of carbon dioxide reduction (CO2RR). In 2016 and 2017, Karen Chan et al. introduced the electric field generated by solvated cations to explain the cation effects on electrochemical CO2RR. Conversely, in 2021, Koper et al. suggested that short-range electrostatic interactions between partially desolvated metal cations and CO2 stabilized CO2 and promoted CO2RR.
Recent researches have combined the exploration of the electrical double layer (EDL) structure with theoretical analysis of PCET kinetics. In 2019, Huang et al. developed a microscopic Hamiltonian model to quantitatively understand the sluggish hydrogen electrocatalysis in alkaline media. In 2021, two meticulous studies from Shao-Horn's group analyzed the effects of cations on reorganization energy and the impacts of hydrogen bonds between proton donors and acceptors on proton tunneling kinetics, respectively. Electrolyte effects on proton transport process were researched in recent years. In 2022, Hu et al. and Chen et al. proposed that the cation-induced electric field distribution and pH-dependent hydrogen bonding network connectivity played essential roles in proton transport, separately.
References
- 1 Fang, W.; Guo, W.; Lu, R.; Yan, Y.; Liu, X.; Wu, D.; Li, F. M.; Zhou, Y.; He, C.; Xia, C.; Niu, H.; Wang, S.; Liu, Y.; Mao, Y.; Zhang, C.; You, B.; Pang, Y.; Duan, L.; Yang, X.; Song, F.; Zhai, T.; Wang, G.; Guo, X.; Tan, B.; Yao, T.; Wang, Z.; Xia, B. Y. Durable CO2 Conversion in the Proton-Exchange Membrane System. Nature 2024, 626, 86–91.
- 2 Lu, Y.; Chen, M.; Wang, Y.; Yang, C.; Zou, Y.; Wang, S. Aqueous Electrocatalytic Small-Molecule Valorization Trilogy. Chem 2024, 10, 1–20.
- 3 Zhong, X. P.; Sui, L. J.; Yang, M. H.; Koketsu, T.; Klingenhof, M.; Selve, S.; Reeves, K. G.; Ge, C. X.; Zhuang, L.; Kan, W. H.; Avdeev, M.; Shu, M.; Alonso-Vante, N.; Chen, J. M.; Haw, S. C.; Pao, C. W.; Chang, Y. C.; Huang, Y. H.; Hu, Z. W.; Strasser, P.; Ma, J. W. Stabilization of Layered Lithium-Rich Manganese Oxide for Anion Exchange Membrane Fuel Cells and Water Electrolysers. Nat. Catal. 2024, 7, 546–559.
- 4 Xie, H.; Zhao, Z.; Liu, T.; Wu, Y.; Lan, C.; Jiang, W.; Zhu, L.; Wang, Y.; Yang, D.; Shao, Z. A Membrane-Based Seawater Electrolyser for Hydrogen Generation. Nature 2022, 612, 673–678.
- 5 Subbaraman, R.; Tripkovic, D.; Strmcnik, D.; Chang, K.-C.; Uchimura, M.; Paulikas, A. P.; Stamenkovic, V.; Markovic, N. M. Enhancing Hydrogen Evolution Activity in Water Splitting by Tailoring Li+-Ni(OH)2-Pt Interfaces. Science 2011, 334, 1256–1260.
- 6 Huang, J. E.; Li, F.; Ozden, A.; Sedighian Rasouli, A.; García de Arquer, F. P.; Liu, S.; Zhang, S.; Luo, M.; Wang, X.; Lum, Y.; Xu, Y.; Bertens, K.; Miao, R. K.; Dinh, C.-T.; Sinton, D.; Sargent, E. H. CO2 Electrolysis to Multicarbon Products in Strong Acid. Science 2021, 372, 1074–1078.
- 7 Lu, X.; Tu, W.; Zhou, Y.; Zou, Z. Effects of Electrolyte Ionic Species on Electrocatalytic Reactions: Advances, Challenges, and Perspectives. Adv. Energy Mater. 2023, 13, 2300628.
- 8 Monteiro, M. C. O.; Dattila, F.; Hagedoorn, B.; García-Muelas, R.; López, N.; Koper, M. T. M. Absence of CO2 Electroreduction on Copper, Gold and Silver Electrodes without Metal Cations in Solution. Nat. Catal. 2021, 4, 654–662.
- 9 Strmcnik, D.; Kodama, K.; van der Vliet, D.; Greeley, J.; Stamenkovic, V. R.; Markovic, N. M. The Role of Non-Covalent Interactions in Electrocatalytic Fuel-Cell Reactions on Platinum. Nat. Chem. 2009, 1, 466–472.
- 10 Chu, A. T.; Surendranath, Y. Aprotic Solvent Exposes an Altered Mechanism for Copper-Catalyzed Ethylene Electrosynthesis. J. Am. Chem. Soc. 2022, 144, 5359–5365.
- 11 Wang, Y.; Zheng, S.; Yang, W.; Zhou, R.; He, Q.; Radjenovic, P.; Dong, J.; Li, S.; Zheng, J.; Yang, Z.; Attard, G.; Pan, F.; Tian, Z.; Li, J. In Situ Raman Spectroscopy Reveals the Structure and Dissociation of Interfacial Water. Nature 2021, 600, 81–85.
- 12 Malkani, A. S.; Li, J.; Oliveira, N. J.; He, M.; Chang, X.; Xu, B.; Lu, Q. Understanding the Electric and Nonelectric Field Components of the Cation Effect on the Electrochemical CO Reduction Reaction. Sci. Adv. 2020, 6, eabd2569.
- 13 Li, P.; Jiang, Y.; Hu, Y.; Men, Y.; Liu, Y.; Cai, W.; Chen, S. Hydrogen Bond Network Connectivity in the Electric Double Layer Dominates the Kinetic pH Effect in Hydrogen Electrocatalysis on Pt. Nat. Catal. 2022, 5, 900–911.
- 14 Li, C.; Le, J.; Wang, Y.; Chen, S.; Yang, Z.; Li, J.; Cheng, J.; Tian, Z. In Situ Probing Electrified Interfacial Water Structures at Atomically Flat Surfaces. Nat. Mater. 2019, 18, 697–701.
- 15 Hou, J.; Xu, B.; Lu, Q. Influence of Electric Double Layer Rigidity on CO Adsorption and Electroreduction Rate. Nat. Commun. 2024, 15, 1926.
- 16 Huang, J.; Li, M.; Eslamibidgoli, M. J.; Eikerling, M.; Groß, A. Cation Overcrowding Effect on the Oxygen Evolution Reaction. JACS Au 2021, 1, 1752–1765.
- 17 Wang, T.; Zhang, Y.; Huang, B.; Cai, B.; Rao, R.; Giordano, L.; Sun, S.; Shao-Horn, Y. Enhancing Oxygen Reduction Electrocatalysis by Tuning Interfacial Hydrogen Bonds. Nat. Catal. 2021, 4, 753–762.
- 18 Waegele, M. M.; Gunathunge, C. M.; Li, J.; Li, X. How Cations Affect the Electric Double Layer and the Rates and Selectivity of Electrocatalytic Processes. J. Chem. Phys. 2019, 151, 160902.
- 19 Sheng, W.; Zhuang, Z.; Gao, M.; Zheng, J.; Chen, J. G.; Yan, Y. Correlating Hydrogen Oxidation and Evolution Activity on Platinum at Different pH with Measured Hydrogen Binding Energy. Nat. Commun. 2015, 6, 5848.
- 20 Zheng, J.; Sheng, W.; Zhuang, Z.; Xu, B.; Yan, Y. Universal Dependence of Hydrogen Oxidation and Evolution Reaction Activity of Platinum-Group Metals on pH and Hydrogen Binding Energy. Sci. Adv. 2016, 2, e1501602.
- 21 Strmcnik, D.; Uchimura, M.; Wang, C.; Subbaraman, R.; Danilovic, N.; van der Vliet, D.; Paulikas, A. P.; Stamenkovic, V. R.; Markovic, N. M. Improving the Hydrogen Oxidation Reaction Rate by Promotion of Hydroxyl Adsorption. Nat. Chem. 2013, 5, 300–306.
- 22 Li, M.; Liao, L.; Yuan, D.; Mei, D.; Chen, Y. pH Effect on Oxygen Reduction Reaction at Pt(111) Electrode. Electrochim. Acta 2013, 110, 780–789.
- 23 Briega-Martos, V.; Herrero, E.; Feliu, J. M. Effect of pH and Water Structure on the Oxygen Reduction Reaction on Platinum Electrodes. Electrochim. Acta 2017, 241, 497–509.
- 24 Fornaciari, J. C.; Weng, L.; Alia, S. M.; Zhan, C.; Pham, T. A.; Bell, A. T.; Ogitsu, T.; Danilovic, N.; Weber, A. Z. Mechanistic Understanding of pH Effects on the Oxygen Evolution Reaction. Electrochim. Acta 2022, 405, 139810.
- 25 Rao, R. R.; Huang, B.; Katayama, Y.; Hwang, J.; Kawaguchi, T.; Lunger, J. R.; Peng, J.; Zhang, Y.; Morinaga, A.; Zhou, H.; You, H.; Shao-Horn, Y. pH- and Cation-Dependent Water Oxidation on Rutile RuO2(110). J. Phys. Chem. C 2021, 125, 8195–8207.
- 26 Huang, B.; Rao, R. R.; You, S.; Hpone Myint, K.; Song, Y.; Wang, Y.; Ding, W.; Giordano, L.; Zhang, Y.; Wang, T.; Muy, S.; Katayama, Y.; Grossman, J. C.; Willard, A. P.; Xu, K.; Jiang, Y.; Shao-Horn, Y. Cation- and pH-Dependent Hydrogen Evolution and Oxidation Reaction Kinetics. JACS Au 2021, 1, 1674–1687.
- 27 Frumkin, A. N.; Nikolaeva-Fedorovich, N. V.; Berezina, N. P.; Keis, K. E. The Electroreduction of the S2O82- Anion. J. Electroanal. Chem. Interfacial Electrochem. 1975, 58, 189–201.
- 28 Chen, L. D.; Urushihara, M.; Chan, K.; Nørskov, J. K. Electric Field Effects in Electrochemical CO2 Reduction. ACS Catal. 2016, 6, 7133–7139.
- 29 Resasco, J.; Chen, L. D.; Clark, E.; Tsai, C.; Hahn, C.; Jaramillo, T. F.; Chan, K.; Bell, A. T. Promoter Effects of Alkali Metal Cations on the Electrochemical Reduction of Carbon Dioxide. J. Am. Chem. Soc. 2017, 139, 11277–11287.
- 30 Gu, J.; Liu, S.; Ni, W.; Ren, W.; Haussener, S.; Hu, X. Modulating Electric Field Distribution by Alkali Cations for CO2 Electroreduction in Strongly Acidic Medium. Nat. Catal. 2022, 5, 268–276.
- 31 Zhang, Z.; Li, H.; Shao, Y.; Gan, L.; Kang, F.; Duan, W.; Hansen, H. A.; Li, J. Molecular Understanding of the Critical Role of Alkali Metal Cations in Initiating CO2 Electroreduction on Cu(100) Surface. Nat. Commun. 2024, 15, 612.
- 32 Singh, M. R.; Kwon, Y.; Lum, Y.; Ager, J. W., III; Bell, A. T. Hydrolysis of Electrolyte Cations Enhances the Electrochemical Reduction of CO2 over Ag and Cu. J. Am. Chem. Soc. 2016, 138, 13006–13012.
- 33 Luo, M.; Koper, M. T. M. A Kinetic Descriptor for the Electrolyte Effect on the Oxygen Reduction Kinetics on Pt(111). Nat. Catal. 2022, 5, 615–623.
- 34 Stamenkovic, V.; Markovic, N. M.; Ross, P. N. Structure-Relationships in Electrocatalysis: Oxygen Reduction and Hydrogen Oxidation Reactions on Pt(111) and Pt(100) in Solutions Containing Chloride Ions. J. Electroanal. Chem. 2001, 500, 44–51.
- 35 Wang, X.; Guo, C.; Zhu, B.; Xiao, D.; Gao, D.; Liu, Z.; Yang, F. Reaction-Induced Iodine Adsorption on Cu Surfaces Facilitates Electrocatalytic CO2 Reduction. J. Chem. Phys. 2023, 158, 204701.
- 36 Sofronov, O. O.; Bakker, H. J. Energy Relaxation and Structural Dynamics of Protons in Water/DMSO Mixtures. J. Phys. Chem. B 2018, 122, 10005–10013.
- 37 Li, S.; Wu, L.; Liu, Q.; Zhu, M.; Li, Z.; Wang, C.; Jiang, X.; Li, J. Uncovering the Dominant Role of an Extended Asymmetric Four-Coordinated Water Network in the Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2023, 145, 26711–26719.
- 38 Xue, S.; Chaudhary, P.; Nouri, M. R.; Gubanova, E.; Garlyyev, B.; Alexandrov, V.; Bandarenka, A. S. Impact of Pt(hkl) Electrode Surface Structure on the Electrical Double Layer Capacitance. J. Am. Chem. Soc. 2024, 146, 3883–3889.
- 39 Gubanova, E.; Schmidt, T. O.; Watzele, S.; Alexandrov, V.; Bandarenka, A. S. Structure-Dependent Electrical Double-Layer Capacitances of the Basal Plane Pd(hkl) Electrodes in HClO4. J. Phys. Chem. C 2022, 126, 11414–11420.
- 40 Chen, A.; Le, J.; Kuang, Y.; Cheng, J. Modeling Stepped Pt/Water Interfaces at Potential of Zero Charge with Ab Initio Molecular Dynamics. J. Chem. Phys. 2022, 157, 094702.
- 41 Strmčnik, D.; Vliet, D. v. d.; Chang, K.-C.; Komanický, V.; Kodama, K.; You, H.; Stamenković, V. R.; Markovic, N. M. Effects of Li+, K+, and Ba2+ Cations on the ORR at Model and High Surface Area Pt and Au Surfaces in Alkaline Solutions. J. Phys. Chem. Lett. 2011, 2, 2733–2736.
- 42 Sitta, E.; Batista, B. C.; Varela, H. The Impact of the Alkali Cation on the Mechanism of the Electro-Oxidation of Ethylene Glycol on Pt. Chem. Commun. 2011, 47, 3775–3777.
- 43 Stoffelsma, C.; Rodriguez, P.; Garcia, G.; Garcia-Araez, N.; Strmcnik, D.; Marković, N. M.; Koper, M. T. M. Promotion of the Oxidation of Carbon Monoxide at Stepped Platinum Single-Crystal Electrodes in Alkaline Media by Lithium and Beryllium Cations. J. Am. Chem. Soc. 2010, 132, 16127–16133.
- 44 Nitopi, S.; Bertheussen, E.; Scott, S. B.; Liu, X.; Engstfeld, A. K.; Horch, S.; Seger, B.; Stephens, I. E. L.; Chan, K.; Hahn, C.; Nørskov, J. K.; Jaramillo, T. F.; Chorkendorff, I. Progress and Perspectives of Electrochemical CO2 Reduction on Copper in Aqueous Electrolyte. Chem. Rev. 2019, 119, 7610–7672.
- 45 Yang, X.; Ding, H.; Li, S.; Zheng, S.; Li, J.-F.; Pan, F. Cation-Induced Interfacial Hydrophobic Microenvironment Promotes the C–C Coupling in Electrochemical CO2 Reduction. J. Am. Chem. Soc. 2024, 146, 5532–5542.
- 46
Helmholtz, H. Ueber Einige Gesetze Der Vertheilung Elektrischer Ströme in Körperlichen Leitern Mit Anwendung Auf Die Thierisch- Elektrischen Versuche. Annalen der Physik 1853, 165, 211–233.
10.1002/andp.18531650603 Google Scholar
- 47 Gouy, M. Sur La Constitution De La Charge Electrique à La Surface D'un Electrolyte. J. Phys. Theor. Appl. 1910, 9, 457–468.
- 48
Chapman, D. L. LI. A Contribution to the Theory of Electrocapillarity. The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science 1913, 25, 475–481.
10.1080/14786440408634187 Google Scholar
- 49 Stern, O. Zur Therorie Der Elektrolytischen Doppelschicht. Zeitschrift für Elektrochemie und angewandte physikalische Chemie 1924, 30, 508–516.
- 50 Grahame, D. C. The Electrical Double Layer and the Theory of Electrocapillarity. Chem. Rev. 1947, 41, 441–501.
- 51 Ovalle, V. J.; Hsu, Y.-S.; Agrawal, N.; Janik, M. J.; Waegele, M. M. Correlating Hydration Free Energy and Specific Adsorption of Alkali Metal Cations During CO2 Electroreduction on Au. Nat. Catal. 2022, 5, 624–632.
- 52 Ringe, S.; Clark, E. L.; Resasco, J.; Walton, A.; Seger, B.; Bell, A. T.; Chan, K. Understanding Cation Effects in Electrochemical CO2 Reduction. Energy Environ. Sci. 2019, 12, 3001–3014.
- 53 Bhattacharyya, D.; Videla, P. E.; Palasz, J. M.; Tangen, I.; Meng, J.; Kubiak, C. P.; Batista, V. S.; Lian, T. Sub-Nanometer Mapping of the Interfacial Electric Field Profile Using a Vibrational Stark Shift Ruler. J. Am. Chem. Soc. 2022, 144, 14330–14338.
- 54 Jiang, T.; Wang, S.; Qin, X.; Zhang, W.; Li, H.; Ma, X.; Jiang, K.; Zou, S.; Cai, W. Uncovering the Cation Effects on the Electroreduction of CO2 on Pd/C Catalysts − an SEIRAS Study. J. Catal. 2024, 434, 115520.
- 55 Ayemoba, O.; Cuesta, A. Spectroscopic Evidence of Size-Dependent Buffering of Interfacial pH by Cation Hydrolysis During CO2 Electroreduction. ACS Appl. Mater. Interfaces 2017, 9, 27377–27382.
- 56 Zhang, F.; Co, A. C. Direct Evidence of Local pH Change and the Role of Alkali Cation During CO2 Electroreduction in Aqueous Media. Angew. Chem. Int. Ed. 2020, 59, 1674–1681.
- 57 Chauhan, P.; Herranz, J.; Winzely, M.; Georgi, M.; Khavlyuk, P.; Eychmüller, A.; Schmidt, T. J. Interfacial pH and Product Selectivity Measurements During CO2 Reduction on a Rotating Ring-Disk Electrode. J. Phys. Chem. C 2023, 127, 16453–16463.
- 58 Malkani, A. S.; Anibal, J.; Xu, B. Cation Effect on Interfacial CO2 Concentration in the Electrochemical CO2 Reduction Reaction. ACS Catal. 2020, 10, 14871–14876.
- 59 Bondue, C. J.; Graf, M.; Goyal, A.; Koper, M. T. M. Suppression of Hydrogen Evolution in Acidic Electrolytes by Electrochemical CO2 Reduction. J. Am. Chem. Soc. 2021, 143, 279–285.
- 60 Sassenburg, M.; Kelly, M.; Subramanian, S.; Smith, W. A.; Burdyny, T. Zero-Gap Electrochemical CO2 Reduction Cells: Challenges and Operational Strategies for Prevention of Salt Precipitation. ACS Energy Lett. 2023, 8, 321–331.
- 61 García de Arquer, F. P.; Dinh, C.-T.; Ozden, A.; Wicks, J.; McCallum, C.; Kirmani, A. R.; Nam, D.-H.; Gabardo, C.; Seifitokaldani, A.; Wang, X.; Li, Y. C.; Li, F.; Edwards, J.; Richter, L. J.; Thorpe, S. J.; Sinton, D.; Sargent, E. H. CO2 Electrolysis to Multicarbon Products at Activities Greater Than 1 A cm-2. Science 2020, 367, 661–666.
- 62 Xie, Y.; Ou, P.; Wang, X.; Xu, Z.; Li, Y. C.; Wang, Z.; Huang, J. E.; Wicks, J.; McCallum, C.; Wang, N.; Wang, Y.; Chen, T.; Lo, B. T. W.; Sinton, D.; Yu, J. C.; Wang, Y.; Sargent, E. H. High Carbon Utilization in CO2 Reduction to Multi-Carbon Products in Acidic Media. Nat. Catal. 2022, 5, 564–570.
- 63 Qin, X.; Hansen, H. A.; Honkala, K.; Melander, M. M. Cation-Induced Changes in the Inner- and Outer-Sphere Mechanisms of Electrocatalytic CO2 Reduction. Nat. Commun. 2023, 14, 7607.
- 64 Ma, W.; Xie, S.; Zhang, X.; Sun, F.; Kang, J.; Jiang, Z.; Zhang, Q.; Wu, D.; Wang, Y. Promoting Electrocatalytic CO2 Reduction to Formate via Sulfur-Boosting Water Activation on Indium Surfaces. Nat. Commun. 2019, 10, 892.
- 65 Yoon, A.; Poon, J.; Grosse, P.; Chee, S. W.; Cuenya, B. R. Iodide-Mediated Cu Catalyst Restructuring During CO2 Electroreduction. J. Mater. Chem. A 2022, 10, 14041–14050.
- 66 Durst, J.; Siebel, A.; Simon, C.; Hasché, F.; Herranz, J.; Gasteiger, H. A. New Insights into the Electrochemical Hydrogen Oxidation and Evolution Reaction Mechanism. Energy Environ. Sci. 2014, 7, 2255–2260.
- 67 Wang, Y.; Wang, X.; Ze, H.; Zhang, X.; Radjenovic, P. M.; Zhang, Y.; Dong, J.; Tian, Z.; Li, J. Spectroscopic Verification of Adsorbed Hydroxy Intermediates in the Bifunctional Mechanism of the Hydrogen Oxidation Reaction. Angew. Chem. Int. Ed. 2021, 60, 5708–5711.
- 68 McCrum, I. T.; Koper, M. T. M. The Role of Adsorbed Hydroxide in Hydrogen Evolution Reaction Kinetics on Modified Platinum. Nat. Energy 2020, 5, 891–899.
- 69 Su, L.; Jin, Y.; Gong, D.; Ge, X.; Zhang, W.; Fan, X.; Luo, W. The Role of Discrepant Reactive Intermediates on Ru-Ru2P Heterostructure for pH-Universal Hydrogen Oxidation Reaction. Angew. Chem. Int. Ed. 2023, 62, e202215585.
- 70 Greeley, J.; Nørskov, J. K.; Kibler, L. A.; El-Aziz, A. M.; Kolb, D. M. Hydrogen Evolution over Bimetallic Systems: Understanding the Trends. ChemPhysChem 2006, 7, 1032–1035.
- 71 Sheng, W.; Myint, M.; Chen, J. G.; Yan, Y. Correlating the Hydrogen Evolution Reaction Activity in Alkaline Electrolytes with the Hydrogen Binding Energy on Monometallic Surfaces. Energy Environ. Sci. 2013, 6, 1509–1512.
- 72 Janik, M. J.; McCrum, I. T.; Koper, M. T. M. On the Presence of Surface Bound Hydroxyl Species on Polycrystalline Pt Electrodes in the “Hydrogen Potential Region” (0–0.4 V-RHE). J. Catal. 2018, 367, 332–337.
- 73 Zhu, S.; Qin, X.; Yao, Y.; Shao, M. pH-Dependent Hydrogen and Water Binding Energies on Platinum Surfaces as Directly Probed through Surface-Enhanced Infrared Absorption Spectroscopy. J. Am. Chem. Soc. 2020, 142, 8748–8754.
- 74 Ledezma-Yanez, I.; Wallace, W. D. Z.; Sebastián-Pascual, P.; Climent, V.; Feliu, J. M.; Koper, M. T. M. Interfacial Water Reorganization as a pH-Dependent Descriptor of the Hydrogen Evolution Rate on Platinum Electrodes. Nat. Energy 2017, 2, 17031.
- 75 Liu, X.; Schlexer, P.; Xiao, J.; Ji, Y.; Wang, L.; Sandberg, R. B.; Tang, M.; Brown, K. S.; Peng, H.; Ringe, S.; Hahn, C.; Jaramillo, T. F.; Nørskov, J. K.; Chan, K. pH Effects on the Electrochemical Reduction of CO(2) Towards C2 Products on Stepped Copper. Nat. Commun. 2019, 10, 32.
- 76 Sun, Q.; Oliveira, N. J.; Kwon, S.; Tyukhtenko, S.; Guo, J. J.; Myrthil, N.; Lopez, S. A.; Kendrick, I.; Mukerjee, S.; Ma, L.; Ehrlich, S. N.; Li, J.; Goddard, W. A.; Yan, Y.; Jia, Q. Understanding Hydrogen Electrocatalysis by Probing the Hydrogen-Bond Network of Water at the Electrified Pt–Solution Interface. Nat. Energy 2023, 8, 859–869.
- 77 Wilson, J. C.; Caratzoulas, S.; Vlachos, D. G.; Yan, Y. Insights into Solvent and Surface Charge Effects on Volmer Step Kinetics on Pt (111). Nat. Commun. 2023, 14, 2384.
- 78 Najm, A. S.; Moria, H.; Ludin, N. A. Areca Catechu as Photovoltaic Sensitizer for Dye-Sensitized Solar Cell (Dssc). Biointerface Res. Appl. Chem. 2020, 10, 5636–5639.
- 79 Tomita, Y.; Teruya, S.; Koga, O.; Hori, Y. Electrochemical Reduction of Carbon Dioxide at a Platinum Electrode in Acetonitrile-Water Mixtures. J. Electrochem. Soc. 2000, 147, 4164–4167.
- 80 Díaz-Duque, Á.; Sandoval-Rojas, A. P.; Molina-Osorio, A. F.; Feliu, J. M.; Suárez-Herrera, M. F. Electrochemical Reduction of CO2 in Water-Acetonitrile Mixtures on Nanostructured Cu Electrode. Electrochem. Commun. 2015, 61, 74–77.
- 81 Figueiredo, M. C.; Ledezma-Yanez, I.; Koper, M. T. M. In Situ Spectroscopic Study of CO2 Electroreduction at Copper Electrodes in Acetonitrile. ACS Catal. 2016, 6, 2382–2392.
- 82 Deacon-Price, C.; da Silva, A. H. M.; Santana, C. S.; Koper, M. T. M.; Garcia, A. C. Solvent Effect on Electrochemical CO2 Reduction Reaction on Nanostructured Copper Electrodes. J. Phys. Chem. C 2023, 127, 14518–14527.
- 83 Amatore, C.; Savéant, J.-M. Mechanism and Kinetic Characteristics of the Electrochemical Reduction of Carbon Dioxide in Media of Low Proton Availability. J. Am. Chem. Soc. 1981, 103, 5021–5023.
- 84 Uemoto, N.; Furukawa, M.; Tateishi, I.; Katsumata, H.; Kaneco, S. Electrochemical Carbon Dioxide Reduction in Methanol at Cu and Cu2O-Deposited Carbon Black Electrodes. ChemEngineering 2019, 3, 15.
- 85 Chu, A. T.; Jung, O.; Toh, W. L.; Surendranath, Y. Organic Non-Nucleophilic Electrolyte Resists Carbonation During Selective CO2 Electroreduction. J. Am. Chem. Soc. 2023, 145, 9617–9623.
- 86 Dubouis, N.; Serva, A.; Berthin, R.; Jeanmairet, G.; Porcheron, B.; Salager, E.; Salanne, M.; Grimaud, A. Tuning Water Reduction through Controlled Nanoconfinement within an Organic Liquid Matrix. Nat. Catal. 2020, 3, 656–663.
- 87 Dorchies, F.; Serva, A.; Sidos, A.; Michot, L.; Deschamps, M.; Salanne, M.; Grimaud, A. Correlating Substrate Reactivity at Electrified Interfaces with the Electrolyte Structure in Synthetically Relevant Organic Solvent/Water Mixtures. J. Am. Chem. Soc. 2024, 146, 17495–17507.
- 88 Bai, X.; Chen, C.; Zhao, X.; Zhang, Y.; Zheng, Y.; Jiao, Y. Accelerating the Reaction Kinetics of CO2 Reduction to Multi-Carbon Products by Synergistic Effect between Cation and Aprotic Solvent on Copper Electrodes. Angew. Chem. Int. Ed. 2024, 63, e202317512.
- 89 Fang, Y.; Fan, Y.; Xie, K.; Ge, W.; Zhu, Y.; Qi, Z.; Song, Z.; Jiang, H.; Li, C. Boosting Hydrogen Peroxide Electrosynthesis Via Modulating the Interfacial Hydrogen-Bond Environment. Angew. Chem. Int. Ed. 2023, 62, e202304413.
- 90 Gomes, R. J.; Kumar, R.; Fejzić, H.; Sarkar, B.; Roy, I.; Amanchukwu, C. V. Modulating Water Hydrogen Bonding within a Non-Aqueous Environment Controls Its Reactivity in Electrochemical Transformations. Nat. Catal. 2024, 7, 689–701.
- 91 Devanathan, M. A. V.; Tilak, B. V. K. S. R. A. The Structure of the Electrical Double Layer at the Metal-Solution Interface. Chem. Rev. 1965, 65, 635–684.
- 92 Le, J.-B.; Chen, A.; Li, L.; Xiong, J.-F.; Lan, J.; Liu, Y.-P.; Iannuzzi, M.; Cheng, J. Modeling Electrified Pt(111)-Had/Water Interfaces from Ab Initio Molecular Dynamics. JACS Au 2021, 1, 569–577.
- 93 Le, J.-B.; Fan, Q.-Y.; Li, J.-Q.; Cheng, J. Molecular Origin of Negative Component of Helmholtz Capacitance at Electrified Pt(111)/Water Interface. Sci. Adv. 2020, 6, eabb1219.
- 94 Le, J.; Cuesta, A.; Cheng, J. The Structure of Metal-Water Interface at the Potential of Zero Charge from Density Functional Theory-Based Molecular Dynamics. J. Electroanal. Chem. 2018, 819, 87–94.
- 95 Wang, T.; Li, L.; Chen, L.; Sheng, T.; Chen, L.; Wang, Y.; Zhang, P.; Hong, Y.; Ye, J.; Lin, W.; Zhang, Q.; Zhang, P.; Fu, G.; Tian, N.; Sun, S.; Zhou, Z. High CO-Tolerant Ru-Based Catalysts by Constructing an Oxide Blocking Layer. J. Am. Chem. Soc. 2022, 144, 9292–9301.
- 96 Goyal, A.; Louisia, S.; Moerland, P.; Koper, M. T. M. Cooperative Effect of Cations and Catalyst Structure in Tuning Alkaline Hydrogen Evolution on Pt Electrodes. J. Am. Chem. Soc. 2024, 146, 7305–7312.
- 97 Xu, X.; Ma, J.; Kui, B.; Zhu, G.; Jia, G.; Wu, F.; Gao, P.; Ye, W. Effect of Crystal Planes of Pd and the Structure of Interfacial Water on the Electrocatalytic Hydrogenation of Alkynes to Alkenes. ACS Appl. Nano Mater. 2023, 6, 5357–5364.
- 98 Xu, A.; Govindarajan, N.; Kastlunger, G.; Vijay, S.; Chan, K. Theories for Electrolyte Effects in CO2 Electroreduction. Acc. Chem. Res. 2022, 55, 495–503.
- 99 Barile, C. J.; Tse, E. C. M.; Li, Y.; Sobyra, T. B.; Zimmerman, S. C.; Hosseini, A.; Gewirth, A. A. Proton Switch for Modulating Oxygen Reduction by a Copper Electrocatalyst Embedded in a Hybrid Bilayer Membrane. Nat. Mater. 2014, 13, 619–623.
- 100 Zhang, X.; Zhao, X.; Zhu, P.; Adler, Z.; Wu, Z.-Y.; Liu, Y.; Wang, H. Electrochemical Oxygen Reduction to Hydrogen Peroxide at Practical Rates in Strong Acidic Media. Nat. Commun. 2022, 13, 2880.
- 101 Tse, E. C. M.; Barile, C. J.; Kirchschlager, N. A.; Li, Y.; Gewargis, J. P.; Zimmerman, S. C.; Hosseini, A.; Gewirth, A. A. Proton Transfer Dynamics Control the Mechanism of O2 Reduction by a Non-Precious Metal Electrocatalyst. Nat. Mater. 2016, 15, 754–759.
- 102 Marcus, R. A. On the Theory of Oxidation-Reduction Reactions Involving Electron Transfer. I. J. Chem. Phys. 1956, 24, 966–978.
- 103 Marcus, R. A. On the Theory of Oxidation-Reduction Reactions Involving Electron Transfer. Ii. Applications to Data on the Rates of Isotopic Exchange Reactions. J. Chem. Phys. 1957, 26, 867–871.
- 104 Marcus, R. A. On the Theory of Electrochemical and Chemical Electron Transfer Processes. Can. J. Chem. 1959, 37, 155–163.
- 105 Li, T. T. T.; Weaver, M. J. Intramolecular Electron Transfer at Metal Surfaces. 4. Dependence of Tunneling Probability Upon Donor-Acceptor Separation Distance. J. Am. Chem. Soc. 1984, 106, 6107–6108.
- 106 Cave, R. J.; Newton, M. D.; Kumar, K.; Zimmt, M. B. Theoretical Study of Solvent Effects on the Electronic Coupling Matrix Element in Rigidly Linked Donor-Acceptor Systems. J. Phys. Chem. C 1995, 99, 17501–17504.
- 107 Troisi, A.; Ratner, M. A.; Zimmt, M. B. Dynamic Nature of the Intramolecular Electronic Coupling Mediated by a Solvent Molecule: A Computational Study. J. Am. Chem. Soc. 2004, 126, 2215–2224.
- 108 Read, I.; Napper, A.; Kaplan, R.; Zimmt, M. B.; Waldeck, D. H. Solvent-Mediated Electronic Coupling: The Role of Solvent Placement. J. Am. Chem. Soc. 1999, 121, 10976–10986.
- 109 Kaplan, R. W.; Napper, A. M.; Waldeck, D. H.; Zimmt, M. B. Solvent Mediated Coupling across 1 nm: not a π Bond in Sight. J. Am. Chem. Soc. 2000, 122, 12039–12040.
- 110 Kumar, K.; Lin, Z.; Waldeck, D. H.; Zimmt, M. B. Electronic Coupling in C-Clamp-Shaped Molecules: Solvent-Mediated Superexchange Pathways. J. Am. Chem. Soc. 1996, 118, 243–244.
- 111 Hildebrandt, A.; Miesel, D.; Yuan, Q.; Freytag, J.; Mahrholdt, J.; Lang, H. Anion and Solvent Dependency of the Electronic Coupling Strength in Mixed Valent Class II Systems. Dalton Trans. 2019, 48, 13162–13168.
- 112 Speck, J. M.; Korb, M.; Hildebrandt, A.; Lang, H. The Role of the Anion in the Charge Transfer Properties of Mixed-Valent Biferrocene. Inorg. Chim. Acta 2018, 483, 39–43.
- 113 Mähler, J.; Persson, I. A Study of the Hydration of the Alkali Metal Ions in Aqueous Solution. Inorg. Chem. 2011, 51, 425–438.
- 114 Ovalle, V. J.; Hsu, Y. S.; Agrawal, N.; Janik, M. J.; Waegele, M. M. Correlating Hydration Free Energy and Specific Adsorption of Alkali Metal Cations During CO2 Electroreduction on Au. Nat. Catal. 2022, 5, 624–632.
- 115 Kim, M.; Park, S.; Chung, T. D. Heterogeneous Electron Transfer Reorganization Energy at the Inner Helmholtz Plane in a Polybromide Redox-Active Ionic Liquid. Chem. Sci. 2022, 13, 8821–8828.
- 116 Huang, B.; Myint, K. H.; Wang, Y.; Zhang, Y.; Rao, R. R.; Sun, J.; Muy, S.; Katayama, Y.; Corchado Garcia, J.; Fraggedakis, D.; Grossman, J. C.; Bazant, M. Z.; Xu, K.; Willard, A. P.; Shao-Horn, Y. Cation-Dependent Interfacial Structures and Kinetics for Outer-Sphere Electron-Transfer Reactions. J. Phys. Chem. C 2021, 125, 4397–4411.
- 117 Huang, B. T.; Myint, K. H.; Wang, Y. M.; Zhang, Y. R.; Rao, R. R.; Sun, J.; Muy, S.; Katayama, Y.; Garcia, J. C.; Fraggedakis, D.; Grossman, J. C.; Bazant, M. Z.; Xu, K.; Willard, A. P.; Shao-Horn, Y. Cation-Dependent Interfacial Structures and Kinetics for Outer-Sphere Electron-Transfer Reactions. J. Phys. Chem. C 2021, 125, 4397–4411.
- 118 Bangle, R. E.; Schneider, J.; Piechota, E. J.; Troian-Gautier, L.; Meyer, G. J. Electron Transfer Reorganization Energies in the Electrode–Electrolyte Double Layer. J. Am. Chem. Soc. 2020, 142, 674–679.
- 119 Reed, S. K.; Madden, P. A.; Papadopoulos, A. Electrochemical Charge Transfer at a Metallic Electrode: A Simulation Study. J. Chem. Phys. 2008, 128, 124701.
- 120 Limaye, A. M.; Ding, W.; Willard, A. P. Understanding Attenuated Solvent Reorganization Energies near Electrode Interfaces. J. Chem. Phys. 2020, 152, 114706.
- 121 Saveant, J. M. A Simple Model for the Kinetics of Dissociative Electron Transfer in Polar Solvents. Application to the Homogeneous and Heterogeneous Reduction of Alkyl Halides. J. Am. Chem. Soc. 1987, 109, 6788–6795.
- 122 Huang, J.; Li, P.; Chen, S. Quantitative Understanding of the Sluggish Kinetics of Hydrogen Reactions in Alkaline Media Based on a Microscopic Hamiltonian Model for the Volmer Step. J. Phys. Chem. C 2019, 123, 17325–17334.
- 123 Huang, J. Mixed Quantum-Classical Treatment of Electron Transfer at Electrocatalytic Interfaces: Theoretical Framework and Conceptual Analysis. J. Chem. Phys. 2020, 153, 164707.
- 124 Turro, C.; Chang, C. K.; Leroi, G. E.; Cukier, R. I.; Nocera, D. G. Photoinduced Electron Transfer Mediated by a Hydrogen-Bonded Interface. J. Am. Chem. Soc. 1992, 114, 4013–4015.
- 125 Roberts, J. A.; Kirby, J. P.; Nocera, D. G. Photoinduced Electron Transfer within a Donor-Acceptor Pair Juxtaposed by a Sal Bridge. J. Am. Chem. Soc. 1995, 117, 8051–8052.
- 126 Soudackov, A.; Hammes-Schiffer, S. Multistate Continuum Theory for Multiple Charge Transfer Reactions in Solution. J. Chem. Phys. 1999, 111, 4672–4687.
- 127 Soudackov, A.; Hammes-Schiffer, S. Derivation of Rate Expressions for Nonadiabatic Proton-Coupled Electron Transfer Reactions in Solution. J. Chem. Phys. 2000, 113, 2385–2396.
- 128 Costentin, C.; Evans, D. H.; Robert, M.; Savéant, J.-M.; Singh, P. S. Electrochemical Approach to Concerted Proton and Electron Transfers. Reduction of the Water−Superoxide Ion Complex. J. Am. Chem. Soc. 2005, 127, 12490–12491.
- 129 Hammes-Schiffer, S. Theory of Proton-Coupled Electron Transfer in Energy Conversion Processes. Acc. Chem. Res. 2009, 42, 1881–1889.
- 130 Ishikita, H.; Soudackov, A. V.; Hammes-Schiffer, S. Buffer-Assisted Proton-Coupled Electron Transfer in a Model Rhenium−Tyrosine Complex. J. Am. Chem. Soc. 2007, 129, 11146–11152.
- 131 Hammes-Schiffer, S.; Stuchebrukhov, A. A. Theory of Coupled Electron and Proton Transfer Reactions. Chem. Rev. 2010, 110, 6939–6960.
- 132 Sayfutyarova, E. R.; Lam, Y.-C.; Hammes-Schiffer, S. Strategies for Enhancing the Rate Constant of C–H Bond Cleavage by Concerted Proton-Coupled Electron Transfer. J. Am. Chem. Soc. 2019, 141, 15183–15189.
- 133 Horvath, S.; Fernandez, L. E.; Soudackov, A. V.; Hammes-Schiffer, S. Insights into Proton-Coupled Electron Transfer Mechanisms of Electrocatalytic H2 Oxidation and Production. Proc. Natl. Acad. Sci U. S. A. 2012, 109, 15663–15668.
- 134 Lam, Y.-C.; Soudackov, A. V.; Hammes-Schiffer, S. Kinetics of Proton Discharge on Metal Electrodes: Effects of Vibrational Nonadiabaticity and Solvent Dynamics. J. Phys. Chem. Lett. 2019, 10, 5312–5317.
- 135 Lam, Y.-C.; Soudackov, A. V.; Hammes-Schiffer, S. Theory of Electrochemical Proton-Coupled Electron Transfer in Diabatic Vibronic Representation: Application to Proton Discharge on Metal Electrodes in Alkaline Solution. J. Phys. Chem. C 2020, 124, 27309–27322.
- 136 Lambros, E.; Link, B.; Chow, M.; Hammes-Schiffer, S.; Li, X. Solvent Induced Proton Polarization within the Nuclear−Electronic Orbital Framework. J. Phys. Chem. Lett. 2023, 14, 2990–2995.
- 137 Jackson, M. N.; Surendranath, Y. Donor-Dependent Kinetics of Interfacial Proton-Coupled Electron Transfer. J. Am. Chem. Soc. 2016, 138, 3228–3234.
- 138 Jackson, M. N.; Jung, O.; Lamotte, H. C.; Surendranath, Y. Donor-Dependent Promotion of Interfacial Proton-Coupled Electron Transfer in Aqueous Electrocatalysis. ACS Catal. 2019, 9, 3737–3743.
- 139 Costentin, C.; Louault, C.; Robert, M.; Savéant, J.-M. The Electrochemical Approach to Concerted Proton-Electron Transfers in the Oxidation of Phenols in Water. Proc. Natl. Acad. Sci. U. S. A. 2009, 106, 18143–18148.
- 140 Costentin, C.; Louault, C.; Robert, M.; Savéant, J.-M. Evidence for Concerted Proton−Electron Transfer in the Electrochemical Oxidation of Phenols with Water as Proton Acceptor. Tri-Tert-Butylphenol. J. Am. Chem. Soc. 2008, 130, 15817–15819.
- 141 Costentin, C.; Louault, C.; Robert, M.; Savéant, J. M. The Electrochemical Approach to Concerted Proton-Electron Transfers in the Oxidation of Phenols in Water. Proc. Natl. Acad. Sci. U. S. A. 2009, 106, 18143–18148.
- 142 Cui, K.; Soudackov, A. V.; Kessinger, M. C.; Xu, J. R. M.; Meyer, G. J.; Hammes-Schiffer, S. General Kinetic Model for pH Dependence of Proton-Coupled Electron Transfer: Application to an Electrochemical Water Oxidation System. J. Am. Chem. Soc. 2023, 145, 19321–19332.
- 143 Cui, K.; Soudackov, A. V.; Hammes-Schiffer, S. Modeling the Weak pH Dependence of Proton-Coupled Electron Transfer for Tryptophan Derivatives. J. Phys. Chem. Lett. 2023, 14, 10980–10987.
- 144 Lewis, N. B.; Bisbey, R. P.; Westendorff, K. S.; Soudackov, A. V.; Surendranath, Y. A Molecular-Level Mechanistic Framework for Interfacial Proton-Coupled Electron Transfer Kinetics. Nat. Chem. 2024, 16, 343–352.
- 145 Cui, K.; Soudackov, A. V.; Kessinger, M. C.; Xu, J.; Meyer, G. J.; Hammes-Schiffer, S. General Kinetic Model for pH Dependence of Proton-Coupled Electron Transfer: Application to an Electrochemical Water Oxidation System. J. Am. Chem. Soc. 2023, 145, 19321–19332.
- 146 Costentin, C.; Drouet, S.; Robert, M.; Savéant, J.-M. A Local Proton Source Enhances CO2 Electroreduction to CO by a Molecular Fe Catalyst. Science 2012, 338, 90–94.
- 147 Costentin, C.; Passard, G.; Robert, M.; Savéant, J.-M. Pendant Acid–Base Groups in Molecular Catalysts: H-Bond Promoters or Proton Relays? Mechanisms of the Conversion of CO2 to CO by Electrogenerated Iron(0)Porphyrins Bearing Prepositioned Phenol Functionalities. J. Am. Chem. Soc. 2014, 136, 11821–11829.
- 148 Savéant, J.-M. Proton Relays in Molecular Catalysis of Electrochemical Reactions: Origin and Limitations of the Boosting Effect. Angew. Chem. Int. Ed. 2019, 58, 2125–2128.
- 149 Deng, K.-C.; Lu, Z.-X.; Sun, J.-J.; Ye, J.-Y.; Dong, F.; Su, H.-S.; Yang, K.; Sartin, M. M.; Yan, S.; Cheng, J.; Zhou, Z.-Y.; Ren, B. Accelerated Interfacial Proton Transfer for Promoting Electrocatalytic Activity. Chem. Sci. 2022, 13, 10884–10890.
- 150 Costentin, C.; Robert, M.; Savéant, J.-M. Concerted Proton–Electron Transfers in the Oxidation of Phenols. Phys. Chem. Chem. Phys. 2010, 12, 11179–11190.
- 151 Li, P.; Jiao, Y.; Ruan, Y.; Fei, H.; Men, Y.; Guo, C.; Wu, Y.; Chen, S. Revealing the Role of Double-Layer Microenvironments in pH-Dependent Oxygen Reduction Activity over Metal-Nitrogen-Carbon Catalysts. Nat. Commun. 2023, 14, 6936.
- 152 Fan, Y.; Chen, Y.; Ge, W.; Dong, L.; Qi, Y.; Lian, C.; Zhou, X.; Liu, H.; Liu, Z.; Jiang, H.; Li, C. Mechanistic Insights into Surfactant-Modulated Electrode–Electrolyte Interface for Steering H2O2 Electrosynthesis. J. Am. Chem. Soc. 2024, 146, 7575–7583.
- 153 Costentin, C.; Robert, M.; Savéant, J.-M. Electrochemical and Homogeneous Proton-Coupled Electron Transfers: Concerted Pathways in the One-Electron Oxidation of a Phenol Coupled with an Intramolecular Amine-Driven Proton Transfer. J. Am. Chem. Soc. 2006, 128, 4552–4553.
- 154 Costentin, C.; Robert, M.; Savéant, J.-M. Adiabatic and Non-Adiabatic Concerted Proton−Electron Transfers. Temperature Effects in the Oxidation of Intramolecularly Hydrogen-Bonded Phenols. J. Am. Chem. Soc. 2010, 132, 2845–2845.
- 155 Clary, K. E.; Karayilan, M.; McCleary-Petersen, K. C.; Petersen, H. A.; Glass, R. S.; Pyun, J.; Lichtenberger, D. L. Increasing the Rate of the Hydrogen Evolution Reaction in Neutral Water with Protic Buffer Electrolytes. Proc. Natl. Acad. Sci. U. S. A. 2020, 117, 32947–32953.
- 156 Brünig, F. N.; Rammler, M.; Adams, E. M.; Havenith, M.; Netz, R. R. Spectral Signatures of Excess-Proton Waiting and Transfer-Path Dynamics in Aqueous Hydrochloric Acid Solutions. Nat. Commun. 2022, 13, 4210.
- 157 Li, X.; Wang, T.; Cai, Y.; Meng, Z.; Nan, J.; Ye, J.; Yi, J.; Zhan, D.; Tian, N.; Zhou, Z.; Sun, S. Mechanism of Cations Suppressing Proton Diffusion Kinetics for Electrocatalysis. Angew. Chem. Int. Ed. 2023, 62, e202218669.




