Nickel/Photoredox Dual Catalytic Chan-Lam Coupling of Aryl Azides and Arylboric Acids
Xia Ge
School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, Jiangsu, 210093 China
These authors contributed equally to this work.
Search for more papers by this authorHaojie Ji
School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, Jiangsu, 210093 China
These authors contributed equally to this work.
Search for more papers by this authorCorresponding Author
Hongjian Lu
School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, Jiangsu, 210093 China
E-mail: [email protected]Search for more papers by this authorXia Ge
School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, Jiangsu, 210093 China
These authors contributed equally to this work.
Search for more papers by this authorHaojie Ji
School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, Jiangsu, 210093 China
These authors contributed equally to this work.
Search for more papers by this authorCorresponding Author
Hongjian Lu
School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, Jiangsu, 210093 China
E-mail: [email protected]Search for more papers by this authorComprehensive Summary
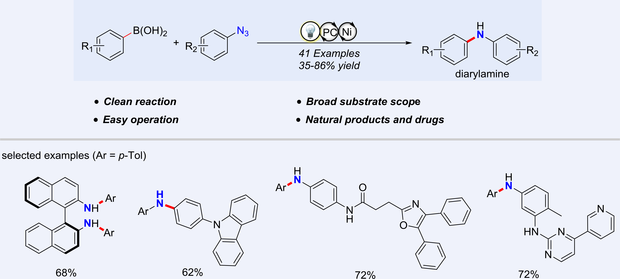
Unsymmetrical diarylamines are crucial components in many pharmaceuticals and functional materials. In this study, we introduce an efficient Chan-Lam cross-coupling method that utilizes phenylboronic acids and aryl azides as coupling agents in a redox-neutral environment, enabled by a synergistic nickel/photoredox catalytic system. This approach leverages a proton-coupled electron transfer mechanism to bypass the typical nitrene pathway associated with aryl azides, which is prone to intramolecular rearrangement, C—H amination, and reductive hydrogenation. Notably, our method exhibits broad compatibility with a variety of functional groups, including those derived from pharmaceuticals, demonstrating its versatile potential in organic synthesis and drug modification.
Supporting Information
| Filename | Description |
|---|---|
| CJOC202400276-sup-0001-supinfo.pdfPDF document, 4 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Ćirić-Marjanović, G. Recent Advances in Polyaniline Research: Polymerization Mechanisms, Structural Aspects, Properties and Applications. Synth. Met. 2013, 177, 1–47; (b) Vitaku, E.; Smith, D. T.; Njardarson, J. T. Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among US FDA Approved Pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274; (c) Ruiz-Castillo, P.; Buchwald, S. L. Applications of Palladium-Catalyzed C–N Cross-Coupling Reactions. Chem. Rev. 2016, 116, 12564–1264.
- 2(a) Vantourout, J. C.; Miras, H. N.; Isidro-Llobet, A.; Sproules, S.; Waston, A. J. B. Spectroscopic Studies of the Chan-Lam Amination: A Mechanism-Inspired Solution to Boronic Ester Reactivity. J. Am. Chem. Soc. 2017, 139, 4769–4779; (b) West, M. J.; Fyfe, J. W. B.; Vantourout, J. C.; Watson, A. J. B. Mechanistic Development and Recent Applications of the Chan-Lam Amination. Chem. Rev. 2019, 119, 12491–12523; (c) Sana, S.; Dastari, S.; Reddy, D. S.; Tokala, R.; Sathish, M.; Sonti, R.; Shankaraiah, N. Visible-light-mediated photocatalytic sequential N-arylation: an eco-friendly synthetic route to unsymmetrical diarylamines and the imatinib drug. Org. Chem. Front. 2023, 10, 4573–4580.
- 3 Munir, I.; Zahoor, A. F.; Rasool, N.; Naqvi, S. A. R.; Zia, K. M.; Ahmad, R. Synthetic applications and methodology development of Chan-Lam coupling: a review. Mol. Divers. 2019, 23, 215–259.
- 4 Xu, S.; Guo, H.; Liu, Y.; Chang, W.; Feng, J.; He, X.; Zhang, Z. Rh(I)-Catalyzed Coupling of Azides with Boronic Acids Under Neutral Conditions. Org. Lett. 2022, 24, 5546–5551.
- 5(a) Tiwari, V. K. Mishra, B. B.; Mishra, K. B.; Mishra, N.; Singh, A. S.; Chen, X. Chem. Rev. 2016, 116, 3086–3240; (b) Kumar, G. S.; Lin, Q. Light-Triggered Click Chemistry. Chem. Rev. 2021, 121, 6991–7031; (c) Fantoni, N. Z.; EI-Sagheer, A. H.; Brown, T. A Hitchhiker's Guide to Click-Chemistry with Nucleic Acids. Chem. Rev. 2021, 121, 7122–7154.
- 6(a) Benati, L.; Bencivenni, G.; Leardini, R.; Minozzi, M.; Nanni, D.; Scialpi, R.; Spagnolo, P.; Zanardi, G. Radical Reduction of Aromatic Azides to Amines with Triethylsilane. J. Org. Chem. 2006, 71, 5822–5825; (b) Pothukanuri, S.; Winssinger, N. A Highly Efficient Azide-Based Protecting Group for Amines and Alcohols. Org. Lett. 2007, 9, 2223–2225; (c) Bartoli, G.; Di Antonio, G.; Giovannini, R.; Giuli, S.; Lanari, S.; Paoletti, M.; Marcantoni, E. Efficient Transformation of Azides to Primary Amines Using the Mild and Easily Accessible CeCl3·7H2O/NaI System. J. Org. Chem. 2008, 73, 1919–1924; (d) Farney, E. P.; Yoon, T. P. Visible-Light Sensitization of Vinyl Azides by Transition-Metal Photocatalysis. Angew. Chem. Int. Ed. 2014, 53, 793−797.
- 7 Meng, G.; Guo, T.; Ma, T.; Zhang, J.; Shen, Y.; Sharpless, K. B.; Dong, J. Modular click chemistry libraries for functional screens using a diazotizing reagent. Nature 2019, 574, 86–89.
- 8(a) Bräse, S.; Gil, C.; Knepper, K.; Zimmermann, V. Organic Azides: An Exploding Diversity of a Unique Class of Compounds. Angew. Chem. Int. Ed. 2005, 44, 5188–5240; (b) Katsuki, T. Azide Compounds: Nitrogen Sources for Atom-efficient and Ecologically Benign Nitrogen-atom-transfer Reactions. Chem. Lett. 2005, 34, 1304–1309; (c) Lu, H.; Zhang, X. P. Catalytic C–H functionalization by metalloporphyrins: recent developments and future directions. Chem. Soc. Rev. 2011, 40, 1899–1909; (d) Driver, T. G. An aminated reaction. Nat. Chem. 2013, 5, 736–738; (f) Shin, K.; Kim, H.; Chang, S. Transition-Metal-Catalyzed C–N Bond Forming Reactions Using Organic Azides as the Nitrogen Source: A Journey for the Mild and Versatile C–H Amination. Acc. Chem. Res. 2015, 48, 1040–1052.
- 9(a) Strieth-Kalthoff, F.; James, M. J.; Teders, M.; Pitzer, L.; Glorius, F. Energy transfer catalysis mediated by visible light: principles, applications, directions. Chem. Soc. Rev. 2018, 47, 7190–7202; (b) Marzo, L.; Pagire, S. K.; Reiser, O.; König, B. Visible-Light Photocatalysis: Does It Make a Difference in Organic Synthesis? Angew. Chem. Int. Ed. 2018, 57, 10034–10072; (c) Buzzetti, L.; Crisenza, G. E. M.; Melchiorre, P. Mechanistic Studies in Photocatalysis. Angew. Chem. Int. Ed. 2019, 58, 3730–3747; (d) Bellotti, P.; Huang, H.; Faber, T.; Glorius, F. Photocatalytic Late-Stage C–H Functionalization. Chem. Rev. 2023, 123, 4237–4352.
- 10For examples of acyl azides as nitrogen sources in photochemistry: (a) Autrey, T.; Schuster, G. B. Are Aroylnitrenes Ground-State Singlets? Photochemistry of ß−NaphthoylAzide. J. Am. Chem. Soc. 1987, 109, 5814–5820; (b) Brachet, E.; Ghosh, T.; Ghosh, I.; König, B. Visible Light C–H Amidation of Heteroarenes with Benzoyl Azides. Chem. Sci. 2015, 6, 987−992; (c) DeBagal, D. B.; Park, S. W.; Song, H. J.; Chang, S. Visible Light Sensitization of Benzoyl Azides: Cascade Cyclization toward Oxindoles via a Non-nitrene Pathway. Chem. Commun. 2017, 53, 8798−8801; (d) Ghosh, T.; Maity, P.; Ranu, B. C. Cu(OAc)2-Promoted Ortho C(sp2)-H Amidation of 8-Aminoquinoline Benzamide with Acyl Azide: Selective Formation of Aroyl or Acetyl Amide Based on Catalyst Loading. J. Org. Chem. 2018, 83, 11758–11767; (e) Bellotti, P.; Brocus, J.; Orf, F. E.; Selkti, M.; König, B.; Belmont, P.; Brachet, E. Visible Light-Induced Regioselective Cycloaddition of Benzoyl Azides and Alkenes to Yield Oxazolines. J. Org. Chem. 2019, 84, 6278–6285.
- 11For examples of azidoformates as nitrogen sources in photochemistry: (a) Lwowski, W.; Mattingly, T. W. The Decomposition of Ethyl Azidoformate in Cyclohexene and in Cyclohexane. J. Am. Chem. Soc. 1965, 87, 1947–1958; (b) Lebel, H.; Piras, H.; Borduy, M. Iron-Catalyzed Amination of Sulfides and Sulfoxides with Azides in Photochemical Continuous Flow Synthesis. ACS Catal. 2016, 6, 1109–1112; (c) Scholz, S. O.; Farney, E. P.; Kim, S.; Bates, D. M.; Yoon, T. P. Spin-Selective Generation of Triplet Nitrenes: Olefin Aziridination through Visible-Light Photosensitization of Azidoformates. Angew. Chem. Int. Ed. 2016, 55, 2239−2242; (d) Zhang, Y.; Dong, X.; Wu, Y.; Li, G.; Lu, H. Visible-Light-Induced Intramolecular C(sp2)-H Amination and Aziridination of Azidoformates via a Triplet Nitrene Pathway. Org. Lett. 2018, 20, 4838−4842; (e) Bouayad-Gervais, S.; Nielsen, C. D.; Tursoy A.; Sperger, T.; Deckers, K.; Schoenebeck, F. Access to Cyclic N-Trifluoromethyl Ureas through Photocatalytic Activation of Carbamoyl Azides. J. Am. Chem. Soc. 2022, 144, 6100–6106.
- 12For examples of sulfonyl azides as nitrogen sources in photochemistry: (a) Hernμndez-Guerra, D.; Hlavackovμ, A.; Pramthaisong, C.; Vespoli, I.; Pohl, R.; Slanina, T.; Jahn, U. Photochemical C–H Amination of Ethers and Geminal Difunctionalization Reactions in One Pot. Angew. Chem. Int. Ed. 2019, 58, 12440–12445; (b) Gui, J.; Xie, H.; Jiang, H.; Zeng, W. Visible-Light-Mediated Sulfonylimination of Tertiary Amines with Sulfonylazides Involving Csp3-Csp3 Bond Cleavage. Org. Lett. 2019, 21, 2804–2807; (c) Ding, R.; Chen, H.; Xu, P.; Tang, H.; Chen, Y.; Pan, Y. Photoinduced Cascade Reaction of Tertiary Amines with Sulfonyl Azides: Synthesis of Amidine Derivatives. Adv. Synth. Catal. 2019, 361, 3656–36; (d) Zhou, S.; Lv, K.; Fu, R.; Zhu, C.; Bao, X., Nickel/Photoredox Dual Catalytic Cross-Coupling of Alkyl and Amidyl Radicals to Construct C(sp3)-N Bonds. ACS Catal. 2021, 11, 5026−5034; (e) Wang, H.; Shao, H.; Haung, G.; Fan, J.; To, W.; Dang, L.; Liu, Y.; Che, M. Chiral Iron Porphyrins Catalyze Enantioselective Intramolecular C(sp3)-H Bond Amination Upon Visible-Light Irradiation. Angew. Chem. Int. Ed. 2023, 62, e202218577.
- 13(a) Konev, M. O.; McTeague, T. A.; Johannes, J. W. Nickel-Catalyzed Photoredox-Mediated Cross-Coupling of Aryl Electrophiles and Aryl Azides. ACS Catal. 2018, 8, 9120−9124; (b) Wu, Y.; Zhang, Y.; Jiang, M.; Dong, X.; Jalani, H. B.; Li, G.; Lu, H. Synergistic combination of visible-light photo-catalytic electron and energy transfer facilitating multicomponent synthesis of β-functionalized α,α-diarylethylamines. Chem. Commun. 2019, 55, 6405−6408; (c) Wu, Y.; Chen, K.; Ge, X.; Ma, P.; Xu, Z.; Lu, H.; Li, G. Redox-Neutral P(O)-N Coupling between P(O)-H Com-pounds and Azides via Dual Copper and Photoredox Catalysis. Org. Lett. 2020, 22, 6143−6149.
- 14(a) Borden, W. T.; Gritsan, N. P.; Hadad, C. M.; Karney, W. L.; Kemnitz, C. R.; Platz, M. S. The Interplay of Theory and Experiment in the Study of Phenylnitrene. Acc. Chem. Res. 2000, 33, 765–771; (b) Gritsan, N. P.; Platz, M. S. Kinetics, Spectroscopy, and Computational Chemistry of Arylnitrenes. Chem. Rev. 2006, 106, 3844−3867.
- 15(a) Patel, S. C.; Burns, N. Z. Conversion of Aryl Azides to Amino-pyridines. J. Am. Chem. Soc. 2022, 144, 17797−17802; (b) Li, G.; Lavagnino, M. N.; Ali, S. Z.; Hu, S.; Radosevich, A. T. Tandem C/N-Difunctionalization of Nitroarenes: Reductive Amination and Annulation by a Ring Expansion/Contraction Sequence. J. Am. Chem. Soc. 2023, 145, 41−46; (c) Matador, E.; Tilby, M. J.; Saridakis, I.; Pedron, M.; Tomczak, D.; Llaveria, J.; Atodiresei, I.; Merino, P.; Ruffoni, A.; Leonori, D. A Photochemical Strategy for the Conversion of Nitroarenes into Rigidiffed Pyrrolidine Analogues. J. Am. Chem. Soc. 2023, 145, 27810−27820; (d) Lai, E. Y.; Guilemard, L. Converting benzene to pyridine. Nat. Rev. Chem. 2023, 7, 71; (e) Li, B.; Ruffoni, A.; Leonori, D. A Photochemical Strategy for ortho-Aminophenol Synthesis via Dearomative-Rearomative Coupling between Aryl Azides and Alcohols. Angew. Chem. Int. Ed. 2023, 62, e202310540.
- 16(a) Xia, X.; Xuan, J.; Wang, Q. L.; Lu, L.; Chen, J.; Xiao, W. Synthesis of 2-Substituted Indoles through Visible Light-Induced Photocatalytic Cyclizations of Styryl Azides. Adv. Synth. Catal. 2014, 356, 2807–2812; (b) Yang, L.; Zhang, Y.; Zou, X.; Lu, H.; Li, G. Visible-light-promoted intramolecular C–H amination in aqueous solution: synthesis of carbazoles. Green Chem. 2018, 20, 1362–1366.
- 17(a) Chen, Y.; Kamlet, A. S.; Steinman, J. B.; Liu, D. R. A biomoleculecompatible visible-light-induced azide reduction from a DNA-encoded reaction-discovery system. Nat. Chem. 2011, 3, 146−153; (b) Vijeta, A.; Casadevall, C.; Reisner, E. An IntegratedCarbon Nitride-Nickel Photocatalyst for the Amination of Aryl Halides Using Sodium Azide. Angew. Chem. Int. Ed. 2022, 61, e202203176.
- 18(a) Zhang, T.; Xu, X.; Dong, X.; Li, G.; Lu, H. Iridium-Catalyzed Unreactive C(sp3)-H Amination with 2,2,2-Trichloroethoxycarbonyl Azide. Org. Lett. 2018, 20, 6260–6264; (b) Zhang, Y.; Ge, X.; Lu, H.; Li, G. Catalytic Decarboxylative C–N Formation to Generate Alkyl, Alkenyl, and Aryl Amines. Angew. Chem. Int. Ed. 2021, 1845 –1852; (c) Lin, K.; Lu, H. DMAP Catalyzed One-Pot Curtius Rearrangement Using 1,1-Dimethyl-2,2,2-trichloroethoxycarbonyl Azide. Org. Lett. 2023, 25, 4534–4539.
- 19(a) Zou, X.; Zou, J.; Yang, L.; Li, G.; Lu, H. Thermal Rearrangement of Sulfamoyl Azides: Reactivity and Mechanistic Study. J. Org. Chem. 2017, 82, 4677–4688; (b) Qin, H.; Cai, W.; Wang, S.; Guo, T.; Li, G.; Lu, H. N-Atom Deletion in Nitrogen Heterocycles. Angew. Chem. Int. Ed. 2021, 60, 20678–20683; (c) Qin, H.; Guo, T.; Lin, K.; Lu, H. Synthesis of dienes from pyrrolidines using skeletal modification. Nat. Commun. 2023, 14, 7307–7314.
- 20(a) Tellis, J. C.; Kelly, C. B.; Primer, D. N.; Jouffroy, M.; Patel, N. R.; Molander, G. A. Single-Electron Transmetalation via Photoredox/Nickel Dual Catalysis: Unlocking a New Paradigm for sp3-sp2 Cross-Coupling. Acc. Chem. Res. 2016, 49, 1429–1439; (b) Prier, C. K.; Rankic, D. A.; MacMillan, D. W. C. Visible Light Photoredox Catalysis with Transition Metal Complexes: Applications in Organic Synthesis. Chem. Rev. 2013, 113, 5322–5363; (c) Cheung, K. P. S.; Sarkar, S.; Gevorgyan, V. Visible Light-Induced Transition Metal Catalysis. Chem. Rev. 2022, 122, 1543–1625.
- 21(a) Gentry, E. C.; Knowles, R. R. Synthetic Applications of Proton-Coupled Electron Transfer. Acc. Chem. Res. 2016, 49, 1546–1556; (b) Murray, P. R. D.; Cox, J. H.; Chiappini, N. D.; Roos, C. B.; McLoughli, E. A.; Hejna, B. G.; Nguyen, S. T.; Ripberger, H. H.; Ganley, J. M.; Tsui, E.; Shin, N. Y.; Koronkiewicz, B.; Qiu, G.; Knowles, R. R. Photochemical and Electrochemical Applications of Proton-Coupled Electron Transfer in Organic Synthesis. Chem. Rev. 2022, 122, 2017−2291.
- 22 Treitler, D. S.; Leung, S. How Dangerous Is Too Dangerous? A Perspective on Azide Chemistry. J. Org. Chem. 2022, 87, 11293–11295.
- 23(a) Ding, K.; Li, X.; Ji, B.; Guo, H.; Kitamura, M. Curr. Org. Synth. 2005, 2, 499−545; (b) Ding, K.; Guo, H.; Li, X.; Yuan, Y.; Wang, Y. Top. Catal. 2005, 35, 105−116.
- 24(a) Tzschucke, C. C.; Murphy, J. M.; Hartwig, J. F. Arenes to Anilines and Aryl Ethers by Sequential Iridium-Catalyzed Borylation and Copper-Catalyzed Coupling. Org. Lett. 2007, 9, 761–764; (b) Vantourout, J. C.; Law, R. P.; Isidro-Llobet, A.; Atkinson, S. J.; Waston, A. J. B. Chan–Evans–Lam Amination of Boronic Acid Pinacol (BPin) Esters: Overcoming the Aryl Amine Problem. J. Org. Chem. 2016, 81, 3942–3950
- 25 Das, S.; Ehlers, A. W.; Patra, S.; Bruin, B. D.; Chattopadhyay, B. Iron-Catalyzed Intermolecular C–N Cross-Coupling Reactions via Radical Activation Mechanism. J. Am. Chem. Soc. 2023, 145, 14599−14607.
- 26(a) Ting, S. I.; Garakyaraghi, S.; Taliaferro, C. M.; Shields, B. J.; Scholes, G. D.; Castellano, F. N.; Doyle, A. G. 3d-d Excited States of Ni(II) Complexes Relevant to Photoredox Catalysis: Spectroscopic Identification and Mechanistic Implications. J. Am. Chem. Soc. 2020, 142, 5800–5810; (b) Diccianni, J. B.; Diao, T. Mechanisms of Nickel-Catalyzed Cross-Coupling Reactions. Trends Chem. 2019, 1, 830−844.
- 27(a) Terrett, J. A.; Cuthberson, J. D.; Shurtleff, V. W.; MacMillan, D. W. C. Switching on elusive organometallic mechanisms with photoredox catalysis. Nature 2015, 524, 330–334;
(b) Na, H.; Mirica, L. M. Deciphering the mechanism of the Ni-photocatalyzed C-O cross-coupling reaction using a tridentate pyridinophane ligand. Nat. Commun. 2022, 14, 1313−1323.
10.1038/s41467-022-28948-8 Google Scholar




