Rhodium-Catalyzed N-Arylation Addition of Arylboronic Acids to Ketimines
Xue-Wei Qian
Department of Chemistry, Fudan University, 220 Handan Road, Shanghai, 200433 China
Search for more papers by this authorCorresponding Author
Xing-Wen Sun
Department of Chemistry, Fudan University, 220 Handan Road, Shanghai, 200433 China
E-mail: [email protected]Search for more papers by this authorXue-Wei Qian
Department of Chemistry, Fudan University, 220 Handan Road, Shanghai, 200433 China
Search for more papers by this authorCorresponding Author
Xing-Wen Sun
Department of Chemistry, Fudan University, 220 Handan Road, Shanghai, 200433 China
E-mail: [email protected]Search for more papers by this authorComprehensive Summary
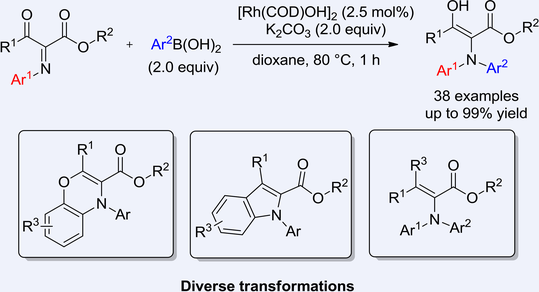
Herein, we report rhodium catalyzed N-arylation via addition of arylboronic acids to electron-deficient α-iminoesters which can be prepared in high efficiency by using easily accessible β-carbonyl esters. The reaction is highly regiospecific to achieve the N-aryl addition efficiently with up to 99% yield under mild conditions. The corresponding product can be further efficiently converted into indoles and a series of other important building blocks.
Supporting Information
| Filename | Description |
|---|---|
| CJOC202400208-sup-0001-supinfo.pdfPDF document, 10.4 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Amino Group Chemistry: From Synthesis to the Life Sciences, Ed.: A. Ricci, Wiley-VCH, Weinheim, 2008, p. 394.
- 2 Hili, R.; Yudin, A. K. Making carbon-nitrogen bonds in biological and chemical synthesis. Nat. Chem. Biol. 2006, 2, 284−287.
- 3 The Chemistry of Anilines, Ed.: Z. Rappoport, Parts 1 and 2, John Wiley & Sons, Chichester, 2007.
- 4 Kürti, L. Streamlining amine synthesis. Science 2015, 348, 863−864.
- 5 Kuriyama, M.; Soeta, T.; Hao, X. Y.; Chen, O.; Tomioka, K. N-Boc-l-Valine-Connected Amidomonophosphane Rhodium(I) Catalyst for Asymmetric Arylation of N-Tosylarylimines with Arylboroxines. J. Am. Chem. Soc. 2004, 126, 8128−8129.
- 6 Tokunaga, N.; Otomaru, Y.; Okamoto, K.; Ueyama, K.; Shintani, R.; Hayashi, T. C2-Symmetric Bicyclo[2.2.2]octadienes as Chiral Ligands: Their High Performance in Rhodium-Catalyzed Asymmetric Arylation of N-Tosylarylimines. J. Am. Chem. Soc. 2004, 126, 13584−13585.
- 7 Duan, H. F.; Jia, Y. X.; Wang, L. X.; Zhou, Q. L. Enantioselective Rh-Catalyzed Arylation of N-Tosylarylimines with Arylboronic Acids. Org. Lett. 2006, 8, 2567−2569.
- 8(a) Trincado, M.; Ellman, J. A. Enantioselective Synthesis of α-Aryl Alkylamines by Rh-Catalyzed Addition Reactions of Arylboronic Acids to Aliphatic Imines. Angew. Chem. Int. Ed. 2008, 47, 5623−5626; (b) Brak, K.; Ellman, J. A. Asymmetric Synthesis of α-Branched Allylic Amines by the Rh(I)-Catalyzed Addition of Alkenyltrifluoroborates to N-tert-Butanesulfinyl Aldimines. J. Am. Chem. Soc. 2009, 131, 3850−3851; (c) Brak, K.; Ellman, J. A. Asymmetric Rh(I)-Catalyzed Addition of MIDA Boronates to N-tert-Butanesulfinyl Aldimines: Development and Comparison to Trifluoroborates. J. Org. Chem. 2010, 75, 3147−3150.
- 9(a) Wang, Z. Q.; Feng, C. G.; Xu, M. H.; Lin, G. Q. Design of C2-Symmetric Tetrahydropentalenes as New Chiral Diene Ligands for Highly Enantioselective Rh-Catalyzed Arylation of N-Tosylarylimines with Arylboronic Acids. J. Am. Chem. Soc. 2007, 129, 5336−5337; (b) Cui, Z.; Yu, H. J.; Yang, R. F.; Gao, W. Y.; Feng, C. G.; Lin, G. Q. Highly Enantioselective Arylation of N-Tosylalkylaldimines Catalyzed by Rhodium-Diene Complexes. J. Am. Chem. Soc. 2011, 133, 12394−12397.
- 10 Wang, H.; Jiang, T.; Xu, M. H. Simple Branched Sulfur–Olefins as Chiral Ligands for Rh-Catalyzed Asymmetric Arylation of Cyclic Ketimines: Highly Enantioselective Construction of Tetrasubstituted Carbon Stereocenters. J. Am. Chem. Soc. 2013, 135, 971−974.
- 11(a) Gopula, B.; Chiang, C. W.; Lee, W. Z.; Kuo, T. S.; Wu, P. Y.; Henschke, J. P.; Wu, H. L. Highly Enantioselective Rh-Catalyzed Alkenylation of Imines: Synthesis of Chiral Allylic Amines via Asymmetric Addition of Potassium Alkenyltrifluoroborates to N-Tosyl Imines. Org Lett. 2014, 16, 632−635; (b) Chiang, P. F.; Li, W. S.; Jian, J. H.; Kuo, T. S.; Wu, P. Y.; Wu, H. L. Rh-Catalyzed Enantioselective Allylation of N-Tosyl- and N-Nosylaldimines: Total Synthesis of (−)-Crispine A. Org Lett. 2018, 20, 158−161; (c) Li, W. S.; Kuo, T. S.; Hsieh, M. C.; Tsai, M. K.; Wu, P. Y.; Wu, H. L. Enantioselective Rhodium-Catalyzed Allylation of Aliphatic Imines: Synthesis of Chiral C-Aliphatic Homoallylic Amines. Org. Lett. 2020, 22, 5675−5679.
- 12
Fiaud, J. C.; Kagan, H. B. Une nouvelle synthese D'α amino-acides. Synthese asymetrique de l'alanine. Tetrahedron Lett. 1970, 11, 1813−1816.
10.1016/S0040-4039(01)98090-6 Google Scholar
- 13 Niwa, Y.; Takayama, K.; Shimizu, M. Electrophilic amination with iminomalonate. Tetrahedron Lett. 2001, 42, 5473−5476.
- 14 Kattamuri, P. V.; Yin, J.; Siriwongsup, S.; Kwon, D. H.; Ess, D. H.; Li, Q.; Li, G.; Yousufuddin, M.; Richardson, P. F.; Sutton, S. C.; Kurti, L. Practical Singly and Doubly Electrophilic Aminating Agents: A New, More Sustainable Platform for Carbon–Nitrogen Bond Formation. J. Am. Chem. Soc. 2017, 139, 11184−11196.
- 15 Sun, Q. S.; Zhu, H.; Chen, Y. J.; Yang, X. D.; Sun, X. W.; Lin, G. Q. Squaramide-Catalyzed Synthesis of Enantioenriched Spirocyclic Oxindoles via Ketimine Intermediates with Multiple Active Sites. Angew. Chem. Int. Ed. 2015, 54, 13253−13257.
- 16For details, please see the Supporting Information.
- 17 Wu, J. H.; Chang, F. R.; Hayashi, K.; Shiraki, H.; Liaw, C. C.; Nakanishi, Y.; Bastow, K. F.; Yu, D. L.; Chen, I. S.; Lee, K. H. Antitumor agents. Part 218: Cappamensin A, a new in vitro anticancer principle, from Capparis sikkimensis. Bioorg. Med. Chem. Lett. 2003, 13, 2223−2225.
- 18 Gawrieh, S.; Noureddin, M.; Loo, N.; Mohseni, R.; Awasty, V.; Cusi, K.; Kowdley, K. V.; Lai, M. C.; Schiff, E.; Parmar, D.; Patel, P; Chalasani, N. Saroglitazar, a PPAR-α/γ Agonist, for Treatment of NAFLD: A Randomized Controlled Double-Blind Phase 2 Trial. Hepatology 2021, 74, 1809−1824.
- 19 Brennan, J. P.; Saxton, J. E. A Total Synthesis of (+/-)-Obscurinervidine. Tetrahedron Lett. 1985, 26, 1769−1772.
- 20 Hayakawa, I.; Atarashi, S.; Yokohama, S.; Imamura, M.; Sakano, K. I.; Furukawa, M. Synthesis and Antibacterial Activities of Optically-Active Ofloxacin. Antimicrob. Agents Chemother. 1986, 29, 163−164.
- 21 Hirayama, T.; Okaniwa, M.; Imada, T.; Ohashi, A.; Ohori, M.; Iwai, K.; Mori, K.; Kawamoto, T.; Yokota, A.; Tanaka, T.; Ishikawa, T. Synthetic studies of centromere-associated Protein-E (CENP-E) inhibitors: 1. Exploration of fused bicyclic core scaffolds using electrostatic potential map. Bioorg. Med. Chem. 2013, 21, 5488−5502.
- 22 Hayashi, T.; Takahashi, M.; Takaya, Y.; Ogasawara, M. Catalytic Cycle of Rhodium-Catalyzed Asymmetric 1,4-Addition of Organoboronic Acids. Arylrhodium, Oxa-π-allylrhodium, and Hydroxorhodium Intermediates. J. Am. Chem. Soc. 2002, 124, 5052−5058.
- 23(a) Dickstein, J. S.; Fennie, M. W.; Norman, A. L.; Paulose, B. J.; Kozlowski, M. C. Three Component Coupling of α-Iminoesters via Umpolung Addition of Organometals: Synthesis of α,α-Disubstituted α-Amino Acids. J. Am. Chem. Soc. 2008, 130, 15794−15795; (b) Curto, J. M.; Dickstein, J. S.; Berritt, S.; Kozlowski, M. C. Asymmetric Synthesis of α-Allyl-α-Aryl α-Amino Acids by Tandem Alkylation/π-Allylation of α-Iminoesters. Org. Lett. 2014, 16, 1948−1951; (c) Curto, J. M.; Kozlowski, M. C. α-Allyl-α-aryl α-Amino Esters in the Asymmetric Synthesis of Acyclic and Cyclic Amino Acid Derivatives by Alkene Metathesis. J. Org. Chem. 2014, 79, 5359−5364.




