Three Types of Isocoumarins with Unusual Carbon Skeletons from Artemisia dubia var. subdigitata and Their Antihepatoma Activity
Ke-Xin Yang
State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, Yunnan, 650201 China
University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorTian-Ze Li
State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, Yunnan, 650201 China
Search for more papers by this authorYun-Bao Ma
State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, Yunnan, 650201 China
Search for more papers by this authorYong-Cui Wang
State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, Yunnan, 650201 China
Search for more papers by this authorFeng-Jiao Li
State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, Yunnan, 650201 China
University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorCorresponding Author
Ji-Jun Chen
State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, Yunnan, 650201 China
University of Chinese Academy of Sciences, Beijing, 100049 China
E-mail: [email protected]Search for more papers by this authorKe-Xin Yang
State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, Yunnan, 650201 China
University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorTian-Ze Li
State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, Yunnan, 650201 China
Search for more papers by this authorYun-Bao Ma
State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, Yunnan, 650201 China
Search for more papers by this authorYong-Cui Wang
State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, Yunnan, 650201 China
Search for more papers by this authorFeng-Jiao Li
State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, Yunnan, 650201 China
University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorCorresponding Author
Ji-Jun Chen
State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, Yunnan, 650201 China
University of Chinese Academy of Sciences, Beijing, 100049 China
E-mail: [email protected]Search for more papers by this authorComprehensive Summary
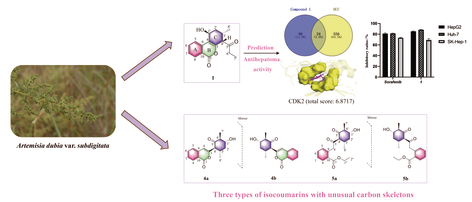
Ten novel isocoumarins, including four pairs of enantiomers, were isolated from Artemisia dubia var. subdigitata (Asteraceae). Compounds 1, 2 and 3a/3b possessed a unique 6/6/6-tricyclic system comprising an unusual 1-(2-methylcyclohexyl) propan-1-one moiety fused with isocoumarin core skeleton. Compounds 4a/4b were characterized as an unexpected 2,5-dimethylcyclohexan-1-one scaffold, and compounds 5a/5b and 6a/6b were rare 1,2-seco-isocoumarin. Their structures and absolute configurations were elucidated through spectroscopic data, X-ray crystallography, ECD and NMR calculations with DP4+ analyses. Plausible biosynthetic pathways were proposed from the naturally occurring isocoumarin. Network pharmacological analysis suggested that the targets of compound 1 were significantly enriched in the cell cycle and PI3K-Akt signaling pathway. The molecular docking revealed that compound 1 had high binding affinity with CDK2 (total score: 6.8717). Furthermore, compounds 1 and 2 exhibited inhibitory activity on three human hepatoma cell lines, with inhibitory ratios of 85.1% and 84.5% (HepG2), 88.2% and 87.3% (Huh7), and 69.2% and 69.1% (SK-Hep-1) at 200 μmol·L–1, respectively.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202400172-sup-0001-supinfo.pdfPDF document, 5.4 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Llovet, J. M.; Kelley, R. K.; Villanueva, A.; Singal, A. G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R. S. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2021, 7, 6.
- 2 Sung, H.; Ferlay, J.; Siegel, R. L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249.
- 3 Alawyia, B.; Constantinou, C. Hepatocellular Carcinoma: a narrative review on current knowledge and future prospects. Curr. Treat. Options. Oncol. 2023, 24, 711–724.
- 4 Luo, X. T.; He, X.; Zhang, X. M.; Zhao, X. H.; Zhang, Y. Z.; Shi, Y. S.; Hua, S. N. Hepatocellular carcinoma: signaling pathways, targeted therapy, and immunotherapy. MedComm 2024, 5, e474.
- 5 Jiang, L.; Li, L.; Liu, Y.; Zhan, M.; Lu, L.; Yuan, S.; Liu, Y. Drug resistance mechanism of kinase inhibitors in the treatment of hepatocellular carcinoma. Front. Pharmacol. 2023, 14, 1097277.
- 6Taskforce; Atanasov, A. G.; Zotchev, S. B.; Dirsch, V. M.; Supuran, C. T. Natural products in drug discovery: advances and opportunities. Nat. Rev. Drug. Discov. 2021, 20, 200–216.
- 7 Saeed, A. Isocoumarins, miraculous natural products blessed with diverse pharmacological activities. Eur. J. Med. Chem. 2016, 116, 290–317.
- 8 Shabir, G.; Saeed, A.; El-Seedi, H. R. Natural isocoumarins: structural styles and biological activities, the revelations carry on …. Phytochemistry 2021, 181, 112568.
- 9 Tammam, M. A.; Gamal El-Din, M. I.; Abood, A.; El-Demerdash, A. Recent advances in the discovery, biosynthesis, and therapeutic potential of isocoumarins derived from fungi: a comprehensive update. RSC Adv. 2023, 13, 8049–8089.
- 10 Chaudhary, N. K.; Pitt, J. I.; Lacey, E.; Crombie, A.; Vuong, D.; Piggott, A. M.; Karuso, P. Banksialactones and banksiamarins: isochromanones and isocoumarins from an Australian fungus, Aspergillus banksianus. J. Nat. Prod. 2018, 81, 1517–1526.
- 11 Kumar, J. S.; Thirupataiah, B.; Medishetti, R.; Ray, A.; Bele, S. D.; Hossain, K. A.; Reddy, G. S.; Edwin, R. K.; Joseph, A.; Kumar, N.; Shenoy, G. G.; Rao, C. M.; Pal, M. Rosuvastatin based novel 3-substituted isocoumarins/3alkylidenephthalides: ultrasound assisted synthesis and identification of new anticancer agents. Eur. J. Med. Chem. 2020, 201, 112335.
- 12 Park, M. J.; Kang, Y.-H. Isolation of isocoumarins and flavonoids as α-glucosidase inhibitors from Agrimonia pilosa L. Molecules 2020, 25, 2572.
- 13 Chen, C.; Ye, G. T.; Tang, J.; Li, J. L.; Liu, W. B.; Wu, L.; Long, Y. H. New polyke-tides from mangrove endophytic fungus Penicillium Sp. BJR-P2 and their anti-inflammatory activity. Mar. Drugs 2022, 20, 583.
- 14 Su, L. H.; Zhang, X.; Ma, Y. B.; Geng, C. A.; Huang, X. Y.; Hu, J.; Li, T. Z; Tang, S.; Shen, C.; Gao, Z.; Zhang, X. M; Chen, J. J. New guaiane-type sesquiterpenoid dimers from Artemisia atrovirens and their antihepatoma activity. Acta Pharm. Sinica B 2021, 11, 1648–1666.
- 15 Feng, X.; Cao, S.; Qiu, F.; Zhang, B. Traditional application and modern pharmacological research of Artemisia annua L. Pharmacol. Therapeut. 2020, 216, 107650.
- 16 He, X. F.; Ma, W. J.; Hu, J.; Li, T. Z.; Geng, C. A.; Ma, Y. B.; Wang, M. F.; Yang, K. X.; Zhang, X. M.; Chen, J. J. Diverse structures and antihepatoma effect of sesquiterpenoid dimers from Artemisia eriopoda by AKT/STAT signaling pathway. Signal. Transduct. Target. Ther. 2023, 8, 64.
- 17 Li, K. M.; Dong, X.; Ma, Y. N.; Wu, Z. H.; Yan, Y. M.; Cheng, Y. X. Antifungal coumarins and lignans from Artemisia annua. Fitoterapia 2019, 134, 323–328.
- 18 Bhutia, T. D.; Valant-Vetschera, K. M. Chemodiversity of Artemisia dracunculus L. from Kyrgyzstan: isocoumarins, coumarins, and flavonoids from aerial parts. Nat. Prod. Commun. 2008, 3, 1289–1292.
- 19
Mallabaev, A.; Sidyakin, G. P. Artemidiol — a new isocoumarin from Artemisia dracunculus. Chem. Nat. Compd. 1974, 10, 743–745.
10.1007/BF00563986 Google Scholar
- 20 Greger, H. Aromatic acetylenes and dehydrofalcarinone derivatives within the Artemisia dracunculus group. Phytochemistry 1979, 18, 1319–1322.
- 21 Lutz-Kutschera, G.; Engelmeier, D.; Hadacek, F.; Werner, A.; Greger, H.; Hofer, O. Synthesis of side chain substituted 3-butylisocoumarins and absolute configurations of natural isocoumarins from Artemisia dracunculus. Monatsh. Chem. 2003, 134, 1195–1206.
- 22 Gao, Z.; Ma, W. J.; Li, T. Z.; Ma, Y. B.; Hu, J.; Huang, X. Y.; Geng, C. A.; He, X. F.; Zhang, X. M.; Chen, J. J. Artemidubolides A-T, cytotoxic unreported guaiane-type sesquiterpenoid dimers against three hepatoma cell lines from Artemisia dubia. Phytochemistry 2022, 202, 113299.
- 23 Gu, W.; Wang, W.; Li, X. N.; Zhang, Y.; Wang, L. P.; Yuan, C. M.; Huang, L. J.; Hao, X. J. A novel isocoumarin with anti-influenza virus activity from Strobilanthes cusia. Fitoterapia 2015, 107, 60–62.
- 24 Grimblat, N.; Zanardi, M. M.; Sarotti, A. M. Beyond DP4: an improved probability for the stereochemical assignment of isomeric compounds using quantum chemical calculations of NMR shifts. J. Org. Chem. 2015, 80, 12526–12534.
- 25 Xu, L.; Guo, F. W.; Zhang, X. Q.; Zhou, T. Y.; Wang, C. J.; Wei, M. Y.; Gu, Y. C.; Wang, C. Y.; Shao, C. L. Discovery, total syntheses and potent anti-inflammatory activity of pyrrolinone-fused benzoazepine alkaloids asperazepanones A and B from Aspergillus candidus. Commun. Chem. 2022, 5, 80.
- 26 Haider, C.; Grubinger, M.; Řezníčková, E.; Weiss, T. S.; Rotheneder, H.; Miklos, W.; Berger, W.; Jorda, R.; Zatloukal, M.; Gucký, T.; Strnad, M.; Kryštof, V.; Mikulits, W. Novel inhibitors of cyclin-dependent kinases combat hepatocellular carcinoma without inducing chemoresistance. Mol. Cancer. Ther. 2013, 12, 1947–1957.
- 27 Sonntag, R.; Giebeler, N.; Nevzorova, Y. A.; Bangen, J. M.; Fahrenkamp, D.; Lambertz, D.; Haas, U.; Hu, W.; Gassler, N.; Cubero, F. J.; Müller-Newen, G.; Abdallah, A. T.; Weiskirchen, R.; Ticconi, F.; Costa, I. G.; Barbacid, M.; Trautwein, C.; Liedtke, C. Cyclin E1 and cyclin-dependent kinase 2 are critical for initiation, but not for progression of hepatocellular carcinoma. Proc. Natl. Acad. Sci. U. S. A. 2018, 115, 9282–9287.
- 28
Farasati Far, B.; Rabie, D.; Hemati, P.; Fooladpanjeh, P.; Faal Hamedanchi, N.; Broomand Lomer, N.; Karimi Rouzbahani, A.; Naimi-Jamal, M. R. Unresectable hepatocellular carcinoma: a review of new advances with focus on targeted therapy and immunotherapy. Livers 2023, 3, 121–160.
10.3390/livers3010011 Google Scholar
- 29 Alden, K.; Read, M.; Timmis, J.; Andrews, P. S.; Veiga-Fernandes, H.; Coles, M. Spartan: A comprehensive tool for understanding uncertainty in simulations of biological systems. PLoS Comput. Biol. 2013, 9, e1002916.
- 30 Zu, W. Y.; Tang, J. W.; Hu, K.; Zhou, Y. F.; Gou, L. L.; Su, X. Z.; Lei, X.; Sun, H. D.; Puno, P. T. Chaetolactam A, an azaphilone derivative from the endophytic fungus Chaetomium sp. g1. J. Org. Chem. 2021, 86, 475–483.
- 31 Willoughby, P. H.; Jansma, M. J.; Hoye, T. R. A guide to small-molecule structure assignment through computation of (1H and 13C) NMR chemical shifts. Nat. Protoc. 2014, 9, 643–660.
- 32 Su, L. H.; Geng, C. A.; Li, T. Z.; Ma, Y. B.; Huang, X. Y.; Zhang, X. M.; Chen, J. J. Artatrovirenols A and B: two cagelike sesquiterpenoids from Artemisia atrovirens. J. Org. Chem. 2020, 85, 13466–13471.
- 33 Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717.
- 34 Gfeller, D.; Grosdidier, A.; Wirth, M.; Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: a web server for target prediction of bioactive small molecules. Nucleic Acids Res. 2014, 42, W32–W38.




