Electrochemical Cascade Annulation for the Synthesis of 3-Sulfanylquinoline Derivatives Under Mild Conditions
Ke Li
State Key Laboratory Base of Eco-Chemical Engineering, College of Chemistry and Molecular Engineering, Qingdao University of Science & Technology, Qingdao, Shandong, 266042 China
Search for more papers by this authorMing-Zhong Guo
State Key Laboratory Base of Eco-Chemical Engineering, College of Chemistry and Molecular Engineering, Qingdao University of Science & Technology, Qingdao, Shandong, 266042 China
Search for more papers by this authorZhuo Chen
State Key Laboratory Base of Eco-Chemical Engineering, College of Chemistry and Molecular Engineering, Qingdao University of Science & Technology, Qingdao, Shandong, 266042 China
Search for more papers by this authorHao-Ran Li
State Key Laboratory Base of Eco-Chemical Engineering, College of Chemistry and Molecular Engineering, Qingdao University of Science & Technology, Qingdao, Shandong, 266042 China
Search for more papers by this authorWeisi Guo
State Key Laboratory Base of Eco-Chemical Engineering, College of Chemistry and Molecular Engineering, Qingdao University of Science & Technology, Qingdao, Shandong, 266042 China
Search for more papers by this authorCorresponding Author
Ming Li
State Key Laboratory Base of Eco-Chemical Engineering, College of Chemistry and Molecular Engineering, Qingdao University of Science & Technology, Qingdao, Shandong, 266042 China
E-mail: [email protected], [email protected]Search for more papers by this authorCorresponding Author
Lin-Bao Zhang
State Key Laboratory Base of Eco-Chemical Engineering, College of Chemistry and Molecular Engineering, Qingdao University of Science & Technology, Qingdao, Shandong, 266042 China
E-mail: [email protected], [email protected]Search for more papers by this authorKe Li
State Key Laboratory Base of Eco-Chemical Engineering, College of Chemistry and Molecular Engineering, Qingdao University of Science & Technology, Qingdao, Shandong, 266042 China
Search for more papers by this authorMing-Zhong Guo
State Key Laboratory Base of Eco-Chemical Engineering, College of Chemistry and Molecular Engineering, Qingdao University of Science & Technology, Qingdao, Shandong, 266042 China
Search for more papers by this authorZhuo Chen
State Key Laboratory Base of Eco-Chemical Engineering, College of Chemistry and Molecular Engineering, Qingdao University of Science & Technology, Qingdao, Shandong, 266042 China
Search for more papers by this authorHao-Ran Li
State Key Laboratory Base of Eco-Chemical Engineering, College of Chemistry and Molecular Engineering, Qingdao University of Science & Technology, Qingdao, Shandong, 266042 China
Search for more papers by this authorWeisi Guo
State Key Laboratory Base of Eco-Chemical Engineering, College of Chemistry and Molecular Engineering, Qingdao University of Science & Technology, Qingdao, Shandong, 266042 China
Search for more papers by this authorCorresponding Author
Ming Li
State Key Laboratory Base of Eco-Chemical Engineering, College of Chemistry and Molecular Engineering, Qingdao University of Science & Technology, Qingdao, Shandong, 266042 China
E-mail: [email protected], [email protected]Search for more papers by this authorCorresponding Author
Lin-Bao Zhang
State Key Laboratory Base of Eco-Chemical Engineering, College of Chemistry and Molecular Engineering, Qingdao University of Science & Technology, Qingdao, Shandong, 266042 China
E-mail: [email protected], [email protected]Search for more papers by this authorComprehensive Summary
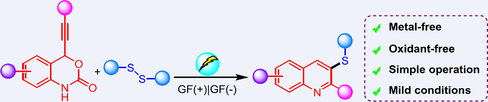
An efficient electrochemical approach has been developed for the construction of 3-sulfanylquinoline derivatives by treating phenylethynylbenzoxazinanones with disulfides in an undivided cell. The protocol provided a convenient route to functionalized quinolines with good functional group tolerance. Moreover, it does not require any metal catalysts or additives, furnishing a series of biological quinolines in moderate to good yields.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202400141-sup-0001-supinfo.pdfPDF document, 3.7 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Beemelmanns, C.; Reissig, H. U. Samarium diiodide induced ketyl-(het)arene cyclisations towards novel N-heterocycles. Chem. Soc. Rev. 2011, 40, 2199–2210; (b) Hancock, R. D. The pyridyl group in ligand design for selective metal ion complexation and sensing. Chem. Soc. Rev. 2013, 42, 1500–1524; (c) Prajapati, S. M.; Patel, K. D.; Vekariya, R. H.; Panchal, S. N.; Patel, H. D. Recent advances in the synthesis of quinolines: a review. RSC Adv. 2014, 4, 24463–24476.
- 2 Vitaku, E.; Smith, D. T.; Njardarson, J. T. Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274.
- 3(a) Michael, J. P. Quinoline, quinazoline and acridone alkaloids. Nat. Prod. Rep. 2008, 25, 166; (b) Anand, N.; Chanda, T.; Koley, S.; Chowdhury, S.; Singh, M. S. CuSO4–D-glucose, an inexpensive and eco-efficient catalytic system: direct access to diverse quinolines through modified Friedlander approach involving SNAr/reduction/ annulation cascade in one pot. RSC Adv. 2015, 5, 7654–7660; (c) Ramann, G. A.; Cowen, B. J. Recent Advances in Metal-Free Quinoline Synthesis. Molecules 2016, 21, 986; (d) Tsoung, J.; Bogdan, A. R.; Kantor, S.; Wang, Y.; Charaschanya, M.; Djuric, S. W. Synthesis of Fused Pyrimidinone and Quinolone Derivatives in an Automated High-Temperature and High-Pressure Flow Reactor. J. Org. Chem. 2017, 82, 1073–1084; (e) da Silva Junior, E. N.; Jardim, G. A. M.; Gomes, R. S.; Liang, Y.-F.; Ackermann, L. Weakly-coordinating N-oxide and carbonyl groups for metal-catalyzed C–H activation: the case of A-ring functionalization. Chem. Commun. 2018, 54, 7398–7411; (f) Weyesa, A.; Mulugeta, E. Recent advances in the synthesis of biologically and pharmaceutically active quinoline and its analogues: a review. RSC Adv. 2020, 10, 20784–20793; (g) Prabagar, B.; Yang, Y.; Shi, Z. Z. Site-selective C–H functionalization to access the arene backbone of indoles and quinolines. Chem. Soc. Rev. 2021, 50, 11249–11269.
- 4 Saari, R.; Törma, J.-C.; Nevalainen, T. Microwave-assisted synthesis of quinoline, isoquinoline, quinoxaline and quinazoline derivatives as CB2 receptor agonists. Bioorg. Med. Chem. 2011, 19, 939–950.
- 5 Boateng, C. A.; Zhu, X. Y.; Jacob, M. R.; Khan, S. I.; Walker, L. A.; Ablordeppey, S. Y. Optimization of 3-(phenylthio)quinolinium compounds against opportunistic fungal pathogens. Eur. J. Med. Chem. 2011, 46, 1789–1797.
- 6(a) Christian, A. H. Metallaphotoredox-Catalyzed C−S Cross-Coupling between Heteroaryl Bromides and α Thioacetic Acids to Access Biaryl Thioethers. J. Org. Chem. 2021, 86, 10914–10920; (b) Qiao, Z. J.; Ge, N. Y.; Jiang, X. F. CO2-promoted oxidative cross-coupling reaction for C–S bond formation via masked strategy in an odourless way. Chem. Commun. 2015, 51, 10295–10298; (c) Liu, B.; Lim, C.-H.; Miyake, G. M. Visible-Light-Promoted C−S Cross-Coupling via Intermolecular Charge Transfer. J. Am. Chem. Soc. 2017, 139, 13616–13619; (d) Lee, J.-Y.; Lee, P. H. Palladium-Catalyzed Carbon-Sulfur Cross-Coupling Reactions with Indium Tri(organothiolate) and Its Application to Sequential One-Pot Processes. J. Org. Chem. 2008, 73, 7413–7416; (e) Cherng, Y.-J. Efficient nucleophilic substitution reactions of quinolyl and isoquinolyl halides with nucleophiles under focused microwave irradiation. Tetrahedron 2002, 58, 1125–1129; (f) Panja, S.; Maity, P.; Kundu, D.; Ranu, B. C. Iron(0) nanoparticles mediated direct conversion of aryl/heteroaryl amines to chalcogenides via in situ diazotization. Tetrahedron Lett. 2017, 58, 3441–3445; (g) Badsara, S. S.; Chan, C.-C.; Lee, C.-F. Transition-Metal-Free Syntheses of Pyridine-Containing Thioethers Through Two-Fold C-S Bond Formation. Asian J. Org. Chem. 2014, 3, 1197–1203; (h) Kimura, T.; Takahashi, N.; Sasage, M.; Namauo, T.; Ogawa, S.; Sato, R. Preparation of pentathiepinoquinoline via ipso-substitution reactions of phenylsulfinyl and iso-propylsulfinyl groups with sulfur anions in liquid ammonia. J. Sulfur Chem. 2009, 30, 377–384; (i) Luo, F.; Pan, C. D.; Li, L. P.; Chen, F.; Cheng, J. Copper-mediated methylthiolation of aryl halides with DMSO. Chem. Commun. 2011, 47, 5304–5306.
- 7 Sheng, X. H.; Yan, M. P.; Zhang, B.; Wong, W.-Y.; Kambe, N.; Qiu, R. H. Nickel-Catalyzed Site-Selective C3−H Functionalization of Quinolines with Electrophilic Reagents at Room Temperature. ACS Catal. 2023, 13, 9753–9765.
- 8 Li, S.; Tang, J.; Fu, Y.-H.; Zheng, X.-L.; Yuan, M.-L.; Li, R.-X.; Su, Z.-S.; Fu, H.-Y.; Chen, H. C3-selective C–H thiolation of quinolines via an N-arylmethyl activation strategy. Org. Chem. Front. 2023, 10, 2324–2331.
- 9(a) Kingston, C.; Palkowitz, M. D.; Takahira, Y.; Vantourout, J. C.; Peters, B. K.; Kawamata, Y.; Baran, P. S. A Survival Guide for the “Electro-curious”. Acc. Chem. Res. 2020, 53, 72–83; (b) Yan, M.; Kawamata, Y.; Baran, P. S. Synthetic Organic Electrochemical Methods Since 2000: On the Verge of a Renaissance. Chem. Rev. 2017, 117, 13230–13319; (c) Novaes, L. F. T.; Liu, J.; Shen, Y.; Lu, L.; Meinhardt, J. M.; Lin, S. Electrocatalysis as an enabling technology for organic synthesis. Chem. Soc. Rev. 2021, 50, 7941–8002; (d) von Münchow, T.; Dana, S.; Xu, Y.; Yuan, B.; Ackermann, L. Enantioselective electrochemical cobalt-catalyzed aryl C–H activation reactions. Science 2023, 379, 1036–1042; (e) Jiao, K. J.; Xing, Y. K.; Yang, Q. L.; Qiu, H.; Mei, T. S. Site-Selective C–H Functionalization via Synergistic Use of Electrochemistry and Transition Metal Catalysis. Acc. Chem. Res. 2020, 53, 300–310; (f) Xiong, P.; Xu, H.-C. Chemistry with Electrochemically Generated N-Centered Radicals. Acc. Chem. Res. 2019, 52, 3339–3350; (g) Malapit, C. A.; Prater, M. B.; Cabrera-Pardo, J. R.; Li, M.; Pham, T. D.; McFadden, T. P.; Blank, S.; Minteer, S. D. Advances on the Merger of Electrochemistry and Transition Metal Catalysis for Organic Synthesis. Chem. Rev. 2022, 122, 3180–3218.
- 10(a) Hioki, Y.; Costantini, M.; Griffin, J.; Harper, K. C.; Merini, M. P.; Nissl, B.; Kawamata, Y.; Baran, P. S. Overcoming the limitations of Kolbe coupling with waveform-controlled electrosynthesis. Science 2023, 380, 81–87; (b) Sun, G. Q.; Yu, P.; Zhang, W.; Zhang, W.; Wang, Y.; Liao, L. L.; Zhang, Z.; Li, L.; Lu, Z.; Yu, D. G.; Lin, S. Electrochemical reactor dictates site selectivity in N-heteroarene carboxylations. Nature 2023, 615, 67–72; (c) Liao, L.-L.; Wang, Z.-H.; Cao, K.-G.; Sun, G.-Q.; Zhang, W.; Ran, C.-K.; Li, Y.; Chen, L.; Cao, G.-M.; Yu, D.-G. Electrochemical Ring-Opening Dicarboxylation of Strained Carbon–Carbon Single Bonds with CO2: Facile Synthesis of Diacids and Derivatization into Polyesters. J. Am. Chem. Soc. 2022, 144, 2062–2068; (d) Jiao, K.-J.; Liu, D.; Ma, H.-X.; Qiu, H.; Fang, P.; Mei, T.-S. Nickel-Catalyzed Electrochemical Reductive Relay Cross-Coupling of Alkyl Halides to Aryl Halides. Angew. Chem. Int. Ed. 2020, 59, 6520–6524; (e) Yang, D. F.; Guan, Z. P.; Peng, Y. A.; Zhu, S. X.; Wang, P. J.; Huang, Z. L.; Alhumade, H.; Gu, D.; Yi, H.; Lei, A. Electrochemical oxidative difunctionalization of diazo compounds with two different nucleophiles. Nat. Commun. 2023, 14, 1476–1484; (f) Gao, Y.; Zhang, B.; He, J.; Baran, P. S. Ni-Electrocatalytic Enantioselective Doubly Decarboxylative C(sp3)–C(sp3) Cross Coupling. J. Am. Chem. Soc. 2023, 145, 11518–11523; (g) Huang, H.; Steiniger, K. A.; Lambert, T. H. Electrophotocatalysis: Combining Light and Electricity to Catalyze Reactions. J. Am. Chem. Soc. 2022, 144, 12567–12583; (h) Hamby, T. B.; LaLama, M. J.; Sevov, C. S. Controlling Ni redox states by dynamic ligand exchange for electroreductive Csp3–Csp2 coupling. Science 2022, 376, 410–416.
- 11 Wang, D.; Zhang, L.; Xiao, F.; Mao, G.; Deng, G. J. The electrochemically selective C3-thiolation of quinolines. Org. Chem. Front. 2022, 9, 2986–2993.
- 12(a) Li, Y.; Wen, L.-R.; Guo, W.-S. A guide to organic electroreduction using sacrificial anodes. Chem. Soc. Rev. 2023, 52, 1168–1188; (b) Fu, Z.-H.; Tian, H.-D.; Ni, S.-F.; Wright, J. S.; Li, M.; Wen, L.-R.; Zhang, L.-B. Scalable selective electrochemical oxidation of sulfides to sulfoxides. Green Chem. 2022, 24, 4772–4777; (c) Li, R.-T.; Yuan, D.-F.; Ping, M.-Q.; Zhu, Y.-Y.; Ni, S.-F.; Li, M.; Wen, L.-R.; Zhang, L.-B. Electrochemically-promoted synthesis of benzo[b]thiophene-1,1-dioxides via strained quaternary spirocyclization. Chem. Sci. 2022, 13, 9940–9946; (d) Zhang, L.-B.; Geng, R.-S.; Wang, Z.-C.; Ren, G.-Y.; Wen, L.-R.; Li, M. Electrochemical intramolecular C–H/N–H functionalization for the synthesis of isoxazolidine-fused isoquinolin-1(2H)-ones. Green Chem. 2020, 22, 16–21; (e) Du, W.-B.; Wang, N.-N.; Pan, C.; Ni, S.-F.; Wen, L.-R.; Li, M.; Zhang, L.-B. Regio- and stereoselective electrochemical synthesis of sulfonylated enethers from alkynes and sulfonyl hydrazides. Green Chem. 2021, 23, 2420–2426; (f) Ping, M.-Q.; Guo, M.-Z.; Li, R.-T.; Wang, Z.-C.; Ma, C.; Wen, L.-R.; Ni, S.-F.; Guo, W.-S.; Li, M.; Zhang, L.-B. Electrochemically Promoted [3 + 2] Annulation of Imidazo[1,2-a]pyridine with Alkynes. Org. Lett. 2022, 24, 7410–7415; (g) Guo, M.-Z.; Mou, M.-J.; Chen, Z.; Ni, S.-F.; Li, M.; Wen, L.-R.; Zhang, L.-B. Electrochemical Reduction of Benzo[b]thiophene 1,1-Dioxides with HFIP as Hydrogen Donor. Chin. J. Chem. 2024, 42, 585–591; (h) Chen, Z.; Li, C.; Liu, K.; Wen, L.-R.; Li, M.; Zhang, L.-B. An electrochemical method for direct sulfonylation of BODIPYs under green conditions. Org. Chem. Front. 2024, 11, 477–483; (i) Li, C.; Chen, Z.; Guo, X.-Y.; Wen, L.-R.; Li, M.; Zhang, L.-B. SO42− ions as a nucleophilic reagent: straightforward electrochemical access to organosulfates. Chem. Commun. 2023, 59, 12164–12167.
- 13(a) Hua, J. W.; Fang, Z.; Xu, J.; Bian, M. X.; Liu, C. K.; He, W.; Zhu, N.; Yang, Z.; Guo, K. Electrochemical oxidative cyclization of activated alkynes with diselenides or disulfides: access to functionalized coumarins or quinolinones. Green Chem. 2019, 21, 4706–4711; (b) Mallick, S.; Baidya, M.; Mahanty, K.; Maiti, D.; Sarkar, S. D. Electrochemical Chalcogenation of β,γ-Unsaturated Amides and Oximes to Corresponding Oxazolines and Isoxazolines. Adv. Synth. Catal. 2020, 362, 1046–1052; (c) Zhang, K.; Wang, J. J.; Wang, X. C.; Zhao, J. C. Electrochemical Oxidative Sulfenocyclization of Alkenoic Acids with Disulfides for the Synthesis of Lactones. Asian J. Org. Chem. 2023, 12, e202300485; (d) Huang, C. F.; Hu, J. J.; Chen, G. X.; Wu, M. J.; Cao, H.; Liu, X. Electrochemical oxidative cyclization of alkenes, boronic acids, and dichalcogenides to access chalcogenated boronic esters and 1,3-diols. Org. Chem. Front. 2022, 9, 12–18.




