Boron-Stereogenic Compounds: Synthetic Developments and Opportunities
Yonghong Guo
Key Laboratory of Medicinal Chemistry and Molecular Diagnosis of the Ministry of Education, Key Laboratory of Chemical Biology of Hebei Province, College of Chemistry and Materials Science, Hebei University, Baoding, Hebei, 071002 China
Search for more papers by this authorBing Zu
Shenzhen Grubbs Institute and Department of Chemistry, Guangdong Provincial Key Laboratory of Catalysis, Southern University of Science and Technology, Shenzhen, Guangdong, 518055 China
Search for more papers by this authorCadmus Du Chen
Shenzhen Grubbs Institute and Department of Chemistry, Guangdong Provincial Key Laboratory of Catalysis, Southern University of Science and Technology, Shenzhen, Guangdong, 518055 China
Search for more papers by this authorCorresponding Author
Chuan He
Shenzhen Grubbs Institute and Department of Chemistry, Guangdong Provincial Key Laboratory of Catalysis, Southern University of Science and Technology, Shenzhen, Guangdong, 518055 China
E-mail: [email protected]Search for more papers by this authorYonghong Guo
Key Laboratory of Medicinal Chemistry and Molecular Diagnosis of the Ministry of Education, Key Laboratory of Chemical Biology of Hebei Province, College of Chemistry and Materials Science, Hebei University, Baoding, Hebei, 071002 China
Search for more papers by this authorBing Zu
Shenzhen Grubbs Institute and Department of Chemistry, Guangdong Provincial Key Laboratory of Catalysis, Southern University of Science and Technology, Shenzhen, Guangdong, 518055 China
Search for more papers by this authorCadmus Du Chen
Shenzhen Grubbs Institute and Department of Chemistry, Guangdong Provincial Key Laboratory of Catalysis, Southern University of Science and Technology, Shenzhen, Guangdong, 518055 China
Search for more papers by this authorCorresponding Author
Chuan He
Shenzhen Grubbs Institute and Department of Chemistry, Guangdong Provincial Key Laboratory of Catalysis, Southern University of Science and Technology, Shenzhen, Guangdong, 518055 China
E-mail: [email protected]Search for more papers by this authorComprehensive Summary
The 21st century has witnessed a continuous evolution in the development of boron-stereogenic chemistry. Since the 1990s, various innovations for the synthesis of tetracoordinate boron-stereogenic compounds, which exhibited great potential applications, have been demonstrated by synthetic chemists. This paper reviews the significant progress and recent advances towards the assembly of enantioenriched boron-stereogenic compounds, and hopes to shed light on new perspectives and inspire further research in this emerging field.
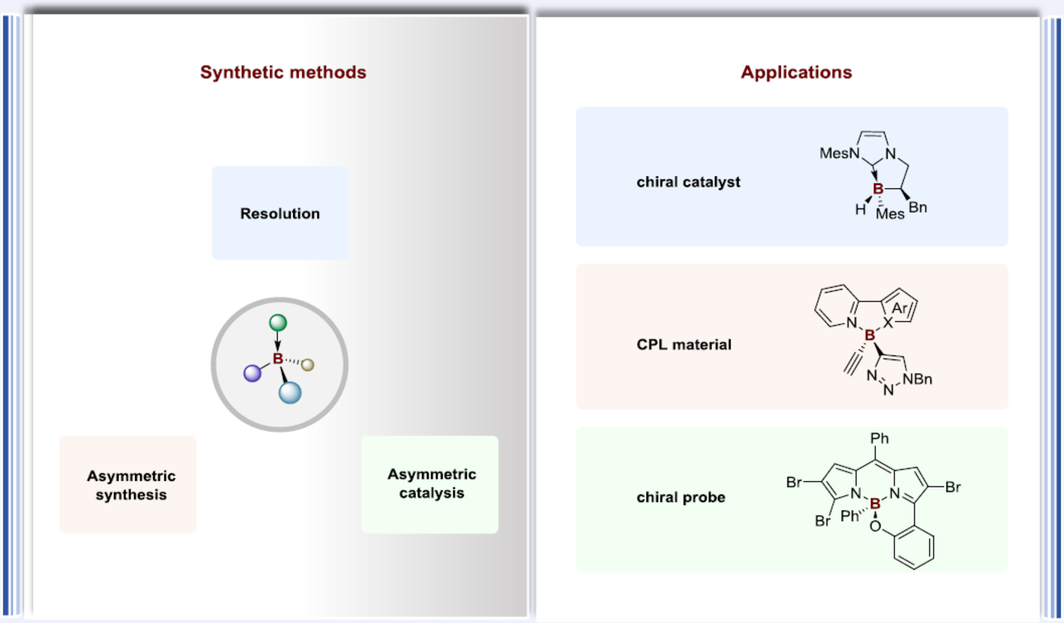
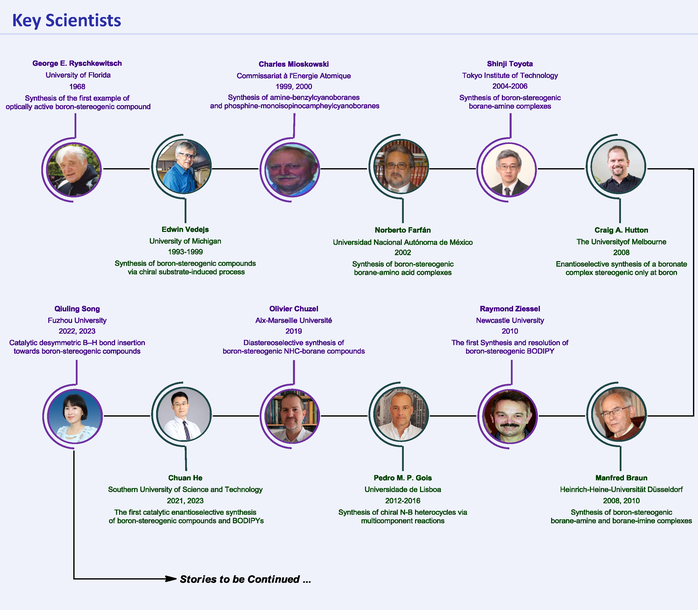
References
- 1Jäkle, F. Boron: Organoboranes. In Encyclopedia of Inorganic Chemistry, Ed.: Crabtree, R. H., John Wiley, Chichester, 2006, pp. 560–598.
- 2 Carboni, B. Synthesis and Application of Organoboron Compounds. Edited by Elena Fernández and Andrew Whiting. Angew. Chem. Int. Ed. 2015, 54, 15010–15011.
- 3 Fyfe, J. W. B.; Watson, A. J. B. Recent Developments in Organoboron Chemistry: Old Dogs, New Tricks. Chem 2017, 3, 31–55.
- 4 Smoum, R.; Rubinstein, A.; Dembitsky, V. M.; Srebnik, M. Boron Containing Compounds as Protease Inhibitors. Chem. Rev. 2012, 112, 4156–4220.
- 5 Yang, F.; Zhu, M.; Zhang, J.; Zhou, H. Synthesis of Biologically Active Boron-Containing Compounds. Med. Chem. Comm. 2018, 9, 201–211.
- 6 Yang, K.; Song, Q. Tetracoordinate Boron Intermediates Enable Unconventional Transformations. Acc. Chem. Res. 2021, 54, 2298–2312.
- 7 Loudet, A.; Burgess, K. BODIPY Dyes and Their Derivatives: Syntheses and Spectroscopic Properties. Chem. Rev. 2007, 107, 4891–4932.
- 8 Ulrich, G.; Ziessel, R.; Harriman, A. The chemistry of Fluorescent Bodipy Dyes: Versatility Unsurpassed. Angew. Chem. Int. Ed. 2008, 47, 1184–1201.
- 9 Staubitz, A.; Robertson, A. P. M.; Sloan, M. E.; Manners, I. Amine- and Phosphine-Borane Adducts: New Interest in Old Molecules. Chem. Rev. 2010, 110, 4023–4078.
- 10 Staubitz, A.; Robertson, A. P. M.; Manners, I. Ammonia-Borane and Related Compounds as Dihydrogen Sources. Chem. Rev. 2010, 110, 4079–4124.
- 11 Curran, D. P.; Solovyev, A.; Brahmi, M. M.; Fensterbank, L.; Malacria, M.; Lacote, E. Synthesis and Reactions of N-Heterocyclic Carbene Boranes. Angew. Chem. Int. Ed. 2011, 50, 10294–10317.
- 12 Li, D.; Zhang, H.; Wang, Y. Four-Coordinate Organoboron Compounds for Organic Light-Emitting Diodes (OLEDs). Chem. Soc. Rev. 2013, 42, 8416–8433.
- 13 Frath, D.; Massue, J.; Ulrich, G.; Ziessel, R. Luminescent Materials: Locking π-Conjugated and Heterocyclic Ligands with Boron(III). Angew. Chem. Int. Ed. 2014, 53, 2290–2310.
- 14 Bruno Prates, J. L.; Pavan, A. R.; dos Santos, J. L. Boron in Medicinal and Organic Chemistry. Curr. Org. Chem. 2021, 25, 1853–1867.
- 15 Trippier, P. C.; McGuigan, C. Boronic Acids in Medicinal Chemistry: Anticancer, Antibacterial and Antiviral Applications. Med. Chem. Commun. 2010, 1, 183–198.
- 16 Suzuki, K.; Kubo, S.; Shizu, K.; Fukushima, T.; Wakamiya, A.; Murata, Y.; Adachi, C.; Kaji, H. Triarylboron-Based Fluorescent Organic Light-Emitting Diodes with External Quantum Efficiencies Exceeding 20%. Angew. Chem. Int. Ed. 2015, 54, 15231–15235.
- 17 Meng, W.; Feng, X.; Du, H. Frustrated Lewis Pairs Catalyzed Asymmetric Metal-Free Hydrogenations and Hydrosilylations. Acc. Chem. Res. 2018, 51, 191–201.
- 18 Leonori, D.; Aggarwal, V. K. Stereospecific Couplings of Secondary and Tertiary Boronic Esters. Angew. Chem. Int. Ed. 2015, 54, 1082–1096.
- 19 Sandford, C.; Aggarwal, V. K. Stereospecific Functionalizations and Transformations of Secondary and Tertiary Boronic Esters. Chem. Commun. 2017, 53, 5481–5494.
- 20 Wang, H.; Jing, C.; Noble, A.; Aggarwal, V. K. Stereospecific 1,2-Migrations of Boronate Complexes Induced by Electrophiles. Angew. Chem. Int. Ed. 2020, 59, 16859–16872.
- 21 Wang, M.; Shi, Z. Methodologies and Strategies for Selective Borylation of C−Het and C−C Bonds. Chem. Rev. 2020, 120, 7348–7398.
- 22 Lu, H.; Mack, J.; Nyokong, T.; Kobayashi, N.; Shen, Z. Optically active BODIPYs. Coord. Chem. Rev. 2016, 318, 1–15.
- 23 Li, X.; Zhang, G.; Song, Q. Recent Advances in the Construction of Tetracoordinate Boron Compounds. Chem. Commun. 2023, 59, 3812–3820.
- 24 Abdou-Mohamed, A.; Aupic, C.; Fournet, C.; Parrain, J. L.; Chouraqui, G.; Chuzel, O. Stereoselective Formation of Boron-Stereogenic Organoboron Derivatives. Chem. Soc. Rev. 2023, 52, 4381–4391.
- 25 Braun, M. Boron-Based Enantiomerism. Eur. J. Org. Chem. 2024, 27, e202400052.
- 26 Maeda, C.; Nagahata, K.; Shirakawa, T.; Ema, T. Azahelicene-Fused BODIPY Analogues Showing Circularly Polarized Luminescence. Angew. Chem. Int. Ed. 2020, 59, 7813–7817.
- 27 Uraguchi, D.; Ueoka, F.; Tanaka, N.; Kizu, T.; Takahashi, W.; Ooi, T. A Structurally Robust Chiral Borate Ion: Molecular Design, Synthesis, and Asymmetric Catalysis. Angew. Chem. Int. Ed. 2020, 59, 11456–11461.
- 28 Zhao, L.; Zhou, H.; Zhou, Q.; Peng, C.; Cheng, T.; Liu, G. Biomimetic Fluorescent Probe for Chiral Glutamic Acid in Water and Its Application in Living Cell Imaging. Sens. Actuators B 2020, 320, 128383.
- 29 Grabulosa, A.; Granell, J.; Muller, G. Preparation of Optically Pure P-Stereogenic Trivalent Phosphorus Compounds. Coord. Chem. Rev. 2007, 251, 25–90.
- 30 Harvey, J. S.; Gouverneur, V. Catalytic Enantioselective Synthesis of P-Stereogenic Compounds. Chem. Commun. 2010, 46, 7477–7485.
- 31 Otocka, S.; Kwiatkowska, M.; Madalinska, L.; Kielbasinski, P. Chiral Organosulfur Ligands/Catalysts with a Stereogenic Sulfur Atom: Applications in Asymmetric Synthesis. Chem. Rev. 2017, 117, 4147–4181.
- 32 Xu, L.-W.; Li, L.; Lai, G.-Q.; Jiang, J.-X. The Recent Synthesis and Application of Silicon-Stereogenic Silanes: A Renewed and Significant Challenge in Asymmetric Synthesis. Chem. Soc. Rev. 2011, 40, 1777–1790.
- 33 Yuan, W.; He, C. Enantioselective C−H Functionalization toward Silicon-Stereogenic Silanes. Synthesis 2022, 54, 1939–1950.
- 34 Zhao, J.; Ge, Y.; He, C. Construction of Silicon-Stereogenic Center via Catalytic Asymmetric Si–H/X–H Dehydrogenative Coupling. Chin. J. Org. Chem. 2023, 43, 3352–3366.
- 35 Schraff, S.; Sun, Y.; Pammer, F. Tuning of Electronic Properties via Labile N→B-Coordination in Conjugated Organoboranes. J. Mater. Chem. C 2017, 5, 1730–1741.
- 36 Mellerup, S. K.; Wang, S. Boron-Based Stimuli Responsive Materials. Chem. Soc. Rev. 2019, 48, 3537–3549.
- 37 Ryschkewitsch, G. E.; Garrett, J. M. Synthesis of Asymmetric Boron Cations and Resolution with As(C6H4P2)3- Anion. J. Am. Chem. Soc. 1968, 90, 7234–7238.
- 38 Charoy, L.; Valleix, A.; Le Gall, T.; Pham van Chuong, P.; Mioskowski, C.; Toupet, L. Synthesis of Benzylcyanoborane Adducts of Amines and Separation of Their Enantiomers; SN2 Substitution at Boron Atom. Chem. Commun. 2000, 2275–2276.
- 39 Toyota, S.; Hakamata, T.; Nitta, N.; Ito, F. Enantiomeric Resolution of Intramolecular Amine-Borane Complex with a Chiral Boron Center. Chem. Lett. 2004, 33, 206–207.
- 40 Toyota, S.; Ito, F.; Nitta, N.; Hakamata, T. Substituent Effects on Configurational Stabilities at Tetrahedral Boron Atoms in Intramolecular Borane-Amine Complexes: Structures, Enantiomeric Resolution, and Rates of Enantiomerization of [2-(Dimethylaminomethyl)phenyl]phenylboranes. Bull. Chem. Soc. Jpn. 2004, 77, 2081–2088.
- 41 Toyota, S.; Ito, F.; Yamamoto, T.; Akashi, H.; Iwanaga, T. Absolute Configuration and Chiroptical Properties of an Enantiopure [5-Chloro-2-(dimethylaminomethyl)phenyl]phenylborane Complex. Bull. Chem. Soc. Jpn. 2006, 79, 796–798.
- 42 Braun, M.; Schlecht, S.; Engelmann, M.; Frank, W.; Grimme, S. Boron-Based Diastereomerism and Enantiomerism in Imine Complexes - Determination of the Absolute Configuration at Boron by CD Spectroscopy. Eur. J. Org. Chem. 2008, 2008, 5221–5225.
- 43 Jimenez, V. G.; Santos, F. M. F.; Castro-Fernandez, S.; Cuerva, J. M.; Gois, P. M. P.; Pischel, U.; Campana, A. G. Circularly Polarized Luminescence of Boronic Acid-Derived Salicylidenehydrazone Complexes Containing Chiral Boron as Stereogenic Unit. J. Org. Chem. 2018, 83, 14057–14062.
- 44 Haefele, A.; Zedde, C.; Retailleau, P.; Ulrich, G.; Ziessel, R. Boron Asymmetry in a BODIPY Derivative. Org. Lett. 2010, 12, 1672–1675.
- 45 Gobo, Y.; Matsuoka, R.; Chiba, Y.; Nakamura, T.; Nabeshima, T. Synthesis and Chiroptical Properties of Phenanthrene-Fused N2O-Type BODIPYs. Tetrahedron Lett. 2018, 59, 4149–4152.
- 46 Imamoto, T.; Morishita, H. An Enantiomerically Pure Tetracoordinate Boron Compound: Stereochemistry of Substitution Reactions at the Chirogenic Boron Atom. J. Am. Chem. Soc. 2000, 122, 6329–6330.
- 47 Vedejs, E.; Fields, S. C.; Schrimpf, M. R. Asymmetric Transformation in Synthesis: Chiral Amino Acid Enolate Equivalents. J. Am. Chem. Soc. 1993, 115, 11612–11613.
- 48 Vedejs, E.; Fields, S. C.; Lin, S.; Schrimpf, M. R. Asymmetric Transformation in Boron Ate Complexes of Amino Acids. J. Org. Chem. 1995, 60, 3028–3034.
- 49 Vedejs, E.; Fields, S. C.; Hayashi, R.; Hitchcock, S. R.; Powell, D. R.; Schrimpf, M. R. Asymmetric Memory at Labile, Stereogenic Boron: Enolate Alkylation of Oxazaborolidinones. J. Am. Chem. Soc. 1999, 121, 2460–2470.
- 50 Vedrenne, P.; Le Guen, V.; Toupet, L.; Le Gall, T.; Mioskowski, C. Synthesis of Diastereomerically Pure Monoisopinocampheylcyanoborane Adducts of Phosphines. Direct Evidence of an SN2 Substitution at a Boron Atom. J. Am. Chem. Soc. 1999, 121, 1090–1091
- 51 Rosendo Rico, A.; Tlahuextl, M.; Flores-Parra, A.; Contreras, R. Addition Reactions of Protonic Reagents to Optically Active 2-Phenyl- 1,3,2-Oxazaborolines. J. Organomet. Chem. 1999, 581, 122–128.
- 52 Beltran, H. I.; Zamudio-Rivera, L. S.; Mancilla, T.; Santillan, R.; Farfán, N. X-Ray Analysis and Structural Characterization of 2-Phenyl-6-Aza- 1,3-Dioxa-2-Borabenzocyclononenones. J. Organomet. Chem. 2002, 657, 194–204.
- 53 Beltran, H. I.; Alas, S. J.; Santillan, R.; Farfan, N. Fixed stereochemical control in the synthesis of new mono- and disubstituted 2-phenyl-6- aza-1,3-dioxa-2-borabenzocyclononenes. Can. J. Chem. 2002, 80, 801–812.
- 54 Kaiser, P. F.; White, J. M.; Hutton, C. A. Enantioselective Preparation of a Stable Boronate Complex Stereogenic Only at Boron. J. Am. Chem. Soc. 2008, 130, 16450–16451.
- 55
Schlecht, S.; Frank, W.; Braun, M. Chelated Boronate-Imine and Boronate-Amine Complexes as Chiral Dopants for Nematic Liquid Crystals. Eur. J. Org. Chem. 2010, 19, 3721–3731.
10.1002/ejoc.201000406 Google Scholar
- 56 Montalbano, F.; Candeias, N. R.; Veiros, L. F.; Andre, V.; Duarte, M. T.; Bronze, M. R.; Moreira, R.; Gois, P. M. P. Four-Component Assembly of Chiral N-B Heterocycles with a Natural Product-Like Framework. Org. Lett. 2012, 14, 988–991.
- 57 Montalbano, F.; Cal, P. M. S. D.; Carvalho, M. A. B. R.; Goncalves, L. M.; Lucas, S. D.; Guedes, R. C.; Veiros, L. F.; Moreira, R.; Gois, P. M. P. Discovery of New Heterocycles with Activity Against Human Neutrophile Elastase Based on a Boron Promoted One-Pot Assembly Reaction. Org. Biomol. Chem. 2013, 11, 4465–4472.
- 58 Montalbano, F.; Leandro, J.; Farias, G. D. V. F.; Lino, P. R.; Guedes, R. C.; Vicente, J. B.; Leandro, P.; Gois, P. M. P. Phenylalanine Iminoboronates as New Phenylalanine Hydroxylase Modulators. RSC Adv. 2014, 4, 61022–61027.
- 59 Faustino, H.; Silva, M. J. S. A.; Veiros, L. F.; Bernardes, G. J. L.; Gois, P. M. P. Iminoboronates are Efficient Intermediates for Selective, Rapid and Reversible N-Terminal Cysteine Functionalisation. Chem. Sci. 2016, 7, 5052–5058.
- 60 Flagstad, T.; Petersen, M. T.; Nielsen, T. E. A Four-Component Reaction for the Synthesis of Dioxadiazaborocines. Angew. Chem. Int. Ed. 2015, 54, 8395–8397.
- 61 Aupic, C.; Abdou Mohamed, A.; Figliola, C.; Nava, P.; Tuccio, B.; Chouraqui, G.; Parrain, J.-L.; Chuzel, O. Highly Diastereoselective Preparation of Chiral NHC-Boranes Stereogenic at the Boron Atom. Chem. Sci. 2019, 10, 6524–6530.
- 62 Algoazy, N.; Knight, J. G.; Waddell, P. G.; Aerts, R.; Herrebout, W.; Al-Sharif, H. H. T.; Karlsson, J. K. G.; Harriman, A. Synthesis, Structure and Photophysical Properties of a New Class of Inherently Chiral Boron(III) Chelates-The tert-Leucine Complexes. Chem. - Eur. J. 2021, 27, 5246–5258.
- 63 Stockl, Y.; Tait, E. J.; Frey, W.; Wegner, S.; Claasen, B.; Zens, A.; Laschat, S. Enantioenriched Boron C,N-Chelates via Chirality Transfer. Chem. Eur. J. 2023, 29, e202301324.
- 64 Ray, C.; Avellanal-Zaballa, E.; Muñoz-Úbeda, M.; Colligan, J.; Moreno, F.; Muller, G.; López-Montero, I.; Bañuelos, J.; Maroto, B. L.; de la Moya, S. Dissimilar-at-Boron N-BODIPYs: From Light-Harvesting MultiChromophoric Arrays to CPL-Bright Chiral-at-Boron BODIPYs. Org. Chem. Front. 2023, 10, 5834–5842.
- 65 Zu, B.; Guo, Y.; He, C. Catalytic Enantioselective Construction of Chiroptical Boron-Stereogenic Compounds. J. Am. Chem. Soc. 2021, 143, 16302–16310.
- 66 Zhang, G.; Zhang, Z.; Hou, M.; Cai, X.; Yang, K.; Yu, P.; Song, Q. Construction of Boron-Stereogenic Compounds via Enantioselective Cu-Catalyzed Desymmetric B-H Bond Insertion Reaction. Nat. Commun. 2022, 13, 2624.
- 67 Zhang, G.; Cai, X.; Jia, J.; Feng, B.; Yang, K.; Song, Q. Cu(I)-Catalyzed Highly Diastereo- and Enantioselective Constructions of Boron/Carbon Vicinal Stereogenic Centers via Insertion Reaction. ACS Catal. 2023, 13, 9502–9508.
- 68 Zu, B.; Guo, Y.; Ren, L.-Q.; Li, Y.; He, C. Catalytic Enantioselective Synthesis of Boron-Stereogenic BODIPYs. Nat. Synth. 2023, 2, 564–571.




