Ruthenium-Catalysed Asymmetric Intramolecular Isomerization/Esterification Reaction: Direct Synthesis of Chiral Dihydrocoumarins
Lingzi Zhao
Frontiers Science Center for Flexible Electronics (FSCFE), Shaanxi Institute of Flexible Electronics (SIFE) & Shaanxi Institute of Biomedical Materials and Engineering (SIBME), Northwestern Polytechnical University (NPU), 127 West Youyi Road, Xi'an, Shaanxi, 710072 China
These authors contributed equally to this work.
Search for more papers by this authorXuchao Wang
Frontiers Science Center for Flexible Electronics (FSCFE), Shaanxi Institute of Flexible Electronics (SIFE) & Shaanxi Institute of Biomedical Materials and Engineering (SIBME), Northwestern Polytechnical University (NPU), 127 West Youyi Road, Xi'an, Shaanxi, 710072 China
These authors contributed equally to this work.
Search for more papers by this authorQing Qiang
Frontiers Science Center for Flexible Electronics (FSCFE), Shaanxi Institute of Flexible Electronics (SIFE) & Shaanxi Institute of Biomedical Materials and Engineering (SIBME), Northwestern Polytechnical University (NPU), 127 West Youyi Road, Xi'an, Shaanxi, 710072 China
Search for more papers by this authorXingwei Zhao
Honors College, Northwestern Polytechnical University (NPU), 127 West Youyi Road, Xi'an, Shaanxi, 710072 China
Search for more papers by this authorCorresponding Author
Feipeng Liu
Frontiers Science Center for Flexible Electronics (FSCFE), Shaanxi Institute of Flexible Electronics (SIFE) & Shaanxi Institute of Biomedical Materials and Engineering (SIBME), Northwestern Polytechnical University (NPU), 127 West Youyi Road, Xi'an, Shaanxi, 710072 China
Email: [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Shenci Lu
Frontiers Science Center for Flexible Electronics (FSCFE), Shaanxi Institute of Flexible Electronics (SIFE) & Shaanxi Institute of Biomedical Materials and Engineering (SIBME), Northwestern Polytechnical University (NPU), 127 West Youyi Road, Xi'an, Shaanxi, 710072 China
Email: [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Zi-Qiang Rong
Frontiers Science Center for Flexible Electronics (FSCFE), Shaanxi Institute of Flexible Electronics (SIFE) & Shaanxi Institute of Biomedical Materials and Engineering (SIBME), Northwestern Polytechnical University (NPU), 127 West Youyi Road, Xi'an, Shaanxi, 710072 China
Email: [email protected]; [email protected]; [email protected]Search for more papers by this authorLingzi Zhao
Frontiers Science Center for Flexible Electronics (FSCFE), Shaanxi Institute of Flexible Electronics (SIFE) & Shaanxi Institute of Biomedical Materials and Engineering (SIBME), Northwestern Polytechnical University (NPU), 127 West Youyi Road, Xi'an, Shaanxi, 710072 China
These authors contributed equally to this work.
Search for more papers by this authorXuchao Wang
Frontiers Science Center for Flexible Electronics (FSCFE), Shaanxi Institute of Flexible Electronics (SIFE) & Shaanxi Institute of Biomedical Materials and Engineering (SIBME), Northwestern Polytechnical University (NPU), 127 West Youyi Road, Xi'an, Shaanxi, 710072 China
These authors contributed equally to this work.
Search for more papers by this authorQing Qiang
Frontiers Science Center for Flexible Electronics (FSCFE), Shaanxi Institute of Flexible Electronics (SIFE) & Shaanxi Institute of Biomedical Materials and Engineering (SIBME), Northwestern Polytechnical University (NPU), 127 West Youyi Road, Xi'an, Shaanxi, 710072 China
Search for more papers by this authorXingwei Zhao
Honors College, Northwestern Polytechnical University (NPU), 127 West Youyi Road, Xi'an, Shaanxi, 710072 China
Search for more papers by this authorCorresponding Author
Feipeng Liu
Frontiers Science Center for Flexible Electronics (FSCFE), Shaanxi Institute of Flexible Electronics (SIFE) & Shaanxi Institute of Biomedical Materials and Engineering (SIBME), Northwestern Polytechnical University (NPU), 127 West Youyi Road, Xi'an, Shaanxi, 710072 China
Email: [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Shenci Lu
Frontiers Science Center for Flexible Electronics (FSCFE), Shaanxi Institute of Flexible Electronics (SIFE) & Shaanxi Institute of Biomedical Materials and Engineering (SIBME), Northwestern Polytechnical University (NPU), 127 West Youyi Road, Xi'an, Shaanxi, 710072 China
Email: [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Zi-Qiang Rong
Frontiers Science Center for Flexible Electronics (FSCFE), Shaanxi Institute of Flexible Electronics (SIFE) & Shaanxi Institute of Biomedical Materials and Engineering (SIBME), Northwestern Polytechnical University (NPU), 127 West Youyi Road, Xi'an, Shaanxi, 710072 China
Email: [email protected]; [email protected]; [email protected]Search for more papers by this authorComprehensive Summary
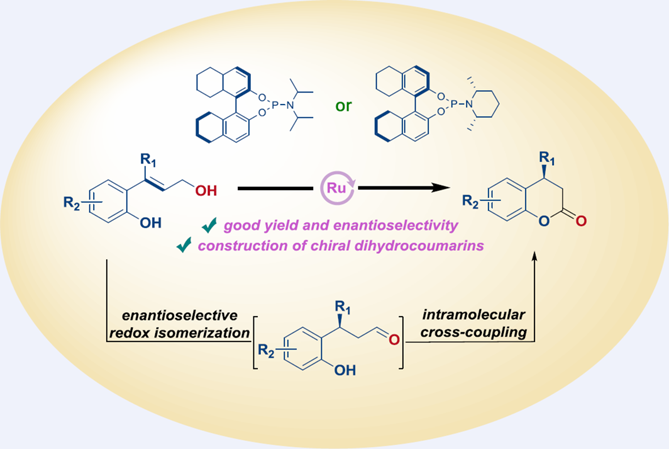
An asymmetric isomerization/intramolecular coupling reaction of allylic alcohols to synthesize chiral dihydrocoumarins was successfully accomplished through ruthenium catalysis. This method demonstrates a wide substrate applicability, excellent tolerance for various functional groups, and good enantioselectivities (up to 90% ee). It provides a convenient pathway to produce a diverse range of structurally distinct chiral dihydrocoumarins in good efficiency.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202400084-sup-0001-supinfo.pdfPDF document, 10.9 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For selected examples, see: (a) Trost, B. M.; Toste, F. D.; Pinkerton, A. B. Non-Metathesis Ruthenium-Catalyzed C−C Bond Formation. Chem. Rev. 2001, 101, 2067−2096; (b) van der Drift, R. C.; Bouwman, E.; Drent, E. Homogeneously Catalysed Isomerisation of Allylic Alcohols to Carbonyl Compounds. J. Organomet. Chem. 2002, 650, 1−24; (c) Uma, R.; Crévisy, C.; Grée, R. Transposition of Allylic Alcohols into Carbonyl Compounds Mediated by Transition Metal Complexes. Chem. Rev. 2003, 103, 27−52; (d) Kuznik, N.; Krompiec, S. Transition Metal Complexes as Catalysts of Double-Bond Migration in O-allyl Systems. Coord. Chem. Rev. 2007, 251, 222−233; (e) Krompiec, S.; Krompiec, M.; Penczek R.; Ignasiak, H. Double Bond Migration in N-Allylic Systems Catalyzed by Transition Metal Complexes. Coord. Chem. Rev. 2008, 252, 1819−1841; (f) Aubert, C.; Fensterbank, L.; Garcia, P.; Malacria, M.; Simonneau, A. Transition Metal Catalyzed Cycloisomerizations of 1,n-Allenynes and -Allenenes. Chem. Rev. 2011, 111, 1954−1993; (g) Mantilli, L.; Mazet, C. Platinum Metals in the Catalytic Asymmetric Isomerization of Allylic Alcohols. Chem. Lett. 2011, 40, 341−344; (h) Lorenzo-Luis, P.; Romerosa, A.; Serrano-Ruiz, M. Catalytic Isomerization of Allylic Alcohols in Water. ACS Catal. 2012, 2, 1079−1086; (i) Ahlsten, N.; Bartoszewicz, A.; Martín-Matute, B. Allylic Alcohols as Synthetic Enolate Equivalents: Isomerisation and Tandem Reactions Catalysed by Transition Metal Complexes. Dalton Trans. 2012, 41, 1660−1670; (j) Cahard, D.; Gaillard, S.; Renaud, J.-L. Asymmetric Isomerization of Allylic Alcohols. Tetrahedron Lett. 2015, 56, 6159−6169; (k) Li, H.; Mazet, C. Iridium-Catalyzed Selective Isomerization of Primary Allylic Alcohols. Acc. Chem. Res. 2016, 49, 1232−1241.
- 2For selected reviews, see: (a) Zhang, X.; Zhang, Y.; Liao, L.; Gao, Y.; Su, H. E. M.; Yu, J. Catalytic Asymmetric Isomerization of (Homo) Allylic Alcohols: Recent Advances and Challenges. ChemCatChem 2022, 14, e202200126; (b) Cadierno, V.; Crochet, P.; Gimeno, J. Ruthenium- Catalyzed Isomerizations of Allylic and Propargylic Alcohols in Aqueous and Organic Media: Applications in Synthesis. Synlett 2008, 2008, 1105–1124; For selected examples see: (c) Mantilli, L.; Gérard, D.; Torche, S.; Besnard, C.; Mazet, C. Iridium-Catalyzed Asymmetric Isomerization of Primary Allylic Alcohols. Angew. Chem. Int. Ed. 2009, 48, 5143–5147; (d) Mantilli, L.; Gérard, D.; Torche, S.; Besnard, C.; Mazet, C. Highly enantioselective isomerization of primary allylic alcohols catalyzed by (P,N)-iridium complexes. Pure Appl. Chem. 2010, 82, 1461–1469; (e) Quintard, A.; Alexakis, A.; Mazet, C. Access to High Levels of Molecular Complexity by One-Pot Iridium/Enamine Asymmetric Catalysis. Angew. Chem. Int. Ed. 2011, 50, 2354–2358; (f) Mazet, C. New Catalytic Asymmetric Strategies to Access Chiral Aldehydes. Chimia 2011, 65, 802–805; (g) Liu, T.-L.; Ng, T. W.; Zhao, Y. Rhodium-Catalyzed Enantioselective Isomerization of Secondary Allylic Alcohols. J. Am. Chem. Soc. 2017, 139, 3643–3646; (h) Huang, R. Z.; Lau, K. K.; Li, Z.; Liu, T. L.; Zhao, Y. Rhodium-Catalyzed Enantioconvergent Isomerization of Homoallylic and Bishomoallylic Secondary Alcohols. J. Am. Chem. Soc. 2018, 140, 14647–14654; (i) Cabré, A.; Garçon, M.; Gallen, A.; Grisoni, L.; Grabulosa, A.; Verdaguer, X.; Riera, A. Iridium-Catalyzed Asymmetric Isomerization of Primary Allylic Alcohols Using MaxPHOX Ligands: Experimental and Theoretical Study. ChemCatChem 2020, 12, 4112–4120; (j) Liu, C.; Yuan, J.; Zhang, Z.; Gridnev, I. D.; Zhang, W. Asymmetric Hydroacylation Involving Alkene Isomerization for the Construction of C3-Chirogenic Center. Angew. Chem. Int. Ed. 2021, 60, 8997–9002; (k) Tani, K. Asymmetric isomerization of allylic compoundsand the mechanism. Pure Appl. Chem. 1985, 57, 1845–1854; (l) Tanaka, K.; Fu, G. C. A Versatile New Catalyst for the Enantioselective Isomerization of Allylic Alcohols to Aldehydes: Scope and Mechanistic Studies. J. Org. Chem. 2001, 66, 8177–8186; (m) Mantilli, L.; Mazet, C. Expanded scope for the iridium-catalyzed asymmetric isomerization of primary allylic alcohols using readily accessible second-generation catalysts. Chem. Commun. 2010, 46, 445–447; (n) Li, H.; Mazet, C. Steric Parameters in the Ir-Catalyzed Regio- and Diastereoselective Isomerization of Primary Allylic Alcohols. Org. Lett. 2013, 15, 6170–6173; (o) Li, H.; Mazet, C. Catalyst-Directed Diastereoselective Isomerization of Allylic Alcohols for the Stereoselective Construction of C(20) in Steroid Side Chains: Scope and Topological Diversification. J. Am. Chem. Soc. 2015, 137, 10720–10727; (p) Li, J. Q.; Peters, B.; Andersson, P. G. Highly Enantioselective Asymmetric Isomerization of Primary Allylic Alcohols with an Iridium–N,P Complex. Chem. Eur. J. 2011, 17, 11143–11145; (q) Ren, K.; Zhang, L.; Hu, B.; Zhao, M.; Tu, Y.; Xie, X.; Zhang, T. Y.; Zhang, Z. Cationic-Rhodium-Catalyzed Kinetic Resolution of Allylic Alcohols through a Redox Isomerization Reaction in a Noncoordinating Solvent. ChemCatChem 2013, 5, 1317–1320; (r) Margalef, J.; Watile, R. A.; Rukkijakan, T.; Samec, J. S. M. High-Atom Economic Approach to Prepare Chiral α-Sulfenylated Ketones. J. Org. Chem. 2019, 84, 11219–11227; (s) Martinez-Erro, S.; Sanz-Marco, A.; Gómez, A. B.; Vázquez-Romero, A. M.; Ahlquist, S. G.; Martín-Matute, B. Base-Catalyzed Stereospecific Isomerization of Electron-Deficient Allylic Alcohols and Ethers through Ion-Pairing. J. Am. Chem. Soc. 2016, 138, 13408–13414; (t) Liu, Y.; Mazet, C. A Catalytic Dual Isomerization/Allylboration Sequence for the Stereoselective Construction of Congested Secondary Homoallylic Alcohols. J. Org. Chem. 2020, 85, 5638–5750.
- 3(a) Quintard, A.; Alexakis, A.; Mazet, C. Access to High Levels of Molecular Complexity by One-Pot Iridium/Enamine Asymmetric Catalysis. Angew. Chem. Int. Ed. 2011, 50, 2354–2358; (b) Liu, Y.; Mazet, C. A Catalytic Dual Isomerization/Allylboration Sequence for the Stereoselective Construction of Congested Secondary Homoallylic Alcohols. J. Org. Chem. 2020, 85, 5638–5650; (c) Arai, N.; Okabe, Y.; Ohkuma, T. Isomerization-Asymmetric Hydrogenation Sequence Converting Racemic β-Ylidenecycloalkanols into Stereocontrolled β-Substituted Cycloalkanols Using a Ru Catalytic System with Dual Roles. Adv. Synth. Catal. 2019, 361, 5540–5547.
- 4For selected reviews, see: (a) Yu, D.; Suzuki, M.; Xie, L.; Morris-Natschke, S.; Lee, K. Recent Progress in the Developmentof Coumarin Derivatives as Potent Anti-HIVAgents. Med. Res. Rev. 2003, 23, 322–345;
(b) Gliszczyńska, A.; Brodelius, P. E. Sesquiterpene coumarins. Phytochem. Rev. 2012, 11, 77–96;
(c) Borges, F.; Roleira, F.; Milhazes, N.; Santana, L.; Uriarte, E. Simple Coumarins and Analogues in Medicinal Chemistry: Occurrence, Synthesis and Biological Activity. Curr. Med. Chem. 2005, 12, 887–916;
(d) Semeniuchenko, V.; Groth, U.; Khilya, V. Synthesis of Chroman-2-ones by Reduction of Coumarins. Synthesis 2009, 2009, 3533–3556.
10.1055/s-0029-1218153 Google Scholar
- 5For selected reviews, see: (a) Kontogiorgis, C.; Hadjipav, D. Synthesis and Antiinflammatory Activity of Coumarin Derivatives. J. Med. Chem. 2005, 48, 6400–6408; (b) Kamat, D.; Tilve, S.; Kamat, V.; Kirtany, J. Syntheses and Biological Activitiesof Chroman-2-ones. A Review. Org. Prep. Proced. Int. 2015, 47, 1–79; (c) Leitis, Z. Synthesis of enantiomerically enriched 4-aryl-3,4-dihydrocoumarins (microreview). Chem. Heterocycl. Compd. 2016, 52, 527–529.
- 6(a) Wang, X.; Liu, F.; Yan, Z.; Qiang, Q.; Huang, W.; Rong, Z.-Q. Redox-Neutral Nickel-Catalyzed Cross-Coupling Reactions of (Homo)allylic Alcohols and Aryltriflates. ACS Catal. 2021, 11, 7319–7326; (b) Yan, Z.; Liu, F.; Wang, X.; Qiang, Q.; Li, Y.; Zhang, Y.; Rong, Z.-Q. Redox-neutral dehydrogenative cross-coupling of alcohols and amines enabled by nickel catalysis. Org. Chem. Front. 2022, 9, 1703–1710; (c) Shui, L.; Liu, F.; Wang, X.; Ma, C.; Qiang, Q.; Shen, M.; Fang, Y.; Ni, S.-F.; Rong, Z.-Q. Ligand-Induced chemodivergent nickel-catalyzed annulations via tandem isomerization/esterification and direct O-allylic substitution: Divergent access to 3,4-dihydrocoumarins and 2H-chromenes. J. Catal. 2023, 421, 264–270.
- 7(a) Wu, R.; Beauchamps, M. G.; Laquidara, J. M.; Sowa Jr, J. R. Ruthenium-Catalyzed Asymmetric Transfer Hydrogenation of Allylic Alcohols by an Enantioselective Isomerization/Transfer Hydrogenation Mechanism. Angew. Chem. Int. Ed. 2012, 51, 2106–2110; (b) Arai, N.; Sato, K.; Azuma, K.; Ohkuma, T. Enantioselective Isomerization of Primary Allylic Alcohols into Chiral Aldehydes with the tol-binap/ dbapen/Ruthenium(II) Catalyst. Angew. Chem. Int. Ed. 2013, 52, 7500–7504; (c) Ríos-Lombardía, N.; Vidal, C.; Liardo, E.; Morís, F.; García-Álvarez, J.; González-Sabín, J. From a Sequential to a Concurrent Reaction in Aqueous Medium: Ruthenium-Catalyzed Allylic Alcohol Isomerization and Asymmetric Bioreduction. Angew. Chem. Int. Ed. 2016, 55, 8691–8695.
- 8 Martín-Matute, B.; Bogár, K.; Edin, M.; Kaynak, F. B.; Bäckvall, J.-E. Highly Efficient Redox Isomerization of Allylic Alcohols at Ambient Temperature Catalyzed by Novel Ruthenium–Cyclopentadienyl Complexes-New Insight into the Mechanism. Chem. Eur. J. 2005, 11, 5832–5842.




