Copper-Mediated Selective Multiple Inert Chemical Bonds Cleavage for Cyanation of Indoles via Tandem Carbon and Nitrogen Atom Transfer
Shimin Xie
Advanced Catalytic Engineering Research Center of the Ministry of Education, College of Chemistry and Chemical Engineering, Hunan University, Changsha, Hunan, 410082 China
Search for more papers by this authorFangfang Cai
Advanced Catalytic Engineering Research Center of the Ministry of Education, College of Chemistry and Chemical Engineering, Hunan University, Changsha, Hunan, 410082 China
Search for more papers by this authorLixin Liu
Advanced Catalytic Engineering Research Center of the Ministry of Education, College of Chemistry and Chemical Engineering, Hunan University, Changsha, Hunan, 410082 China
Search for more papers by this authorLebin Su
Advanced Catalytic Engineering Research Center of the Ministry of Education, College of Chemistry and Chemical Engineering, Hunan University, Changsha, Hunan, 410082 China
Search for more papers by this authorCorresponding Author
Jianyu Dong
School of Physics and Chemistry, Hunan First Normal University, Changsha, Hunan, 410205 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Yongbo Zhou
Advanced Catalytic Engineering Research Center of the Ministry of Education, College of Chemistry and Chemical Engineering, Hunan University, Changsha, Hunan, 410082 China
E-mail: [email protected]; [email protected]Search for more papers by this authorShimin Xie
Advanced Catalytic Engineering Research Center of the Ministry of Education, College of Chemistry and Chemical Engineering, Hunan University, Changsha, Hunan, 410082 China
Search for more papers by this authorFangfang Cai
Advanced Catalytic Engineering Research Center of the Ministry of Education, College of Chemistry and Chemical Engineering, Hunan University, Changsha, Hunan, 410082 China
Search for more papers by this authorLixin Liu
Advanced Catalytic Engineering Research Center of the Ministry of Education, College of Chemistry and Chemical Engineering, Hunan University, Changsha, Hunan, 410082 China
Search for more papers by this authorLebin Su
Advanced Catalytic Engineering Research Center of the Ministry of Education, College of Chemistry and Chemical Engineering, Hunan University, Changsha, Hunan, 410082 China
Search for more papers by this authorCorresponding Author
Jianyu Dong
School of Physics and Chemistry, Hunan First Normal University, Changsha, Hunan, 410205 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Yongbo Zhou
Advanced Catalytic Engineering Research Center of the Ministry of Education, College of Chemistry and Chemical Engineering, Hunan University, Changsha, Hunan, 410082 China
E-mail: [email protected]; [email protected]Search for more papers by this authorComprehensive Summary
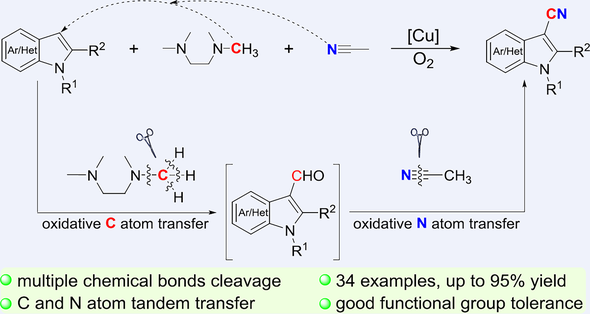
The activation of inert chemical bonds is an exciting area of research in chemistry because it enables the direct utilization of readily available starting materials and promotes atom- and step-economic synthesis. Undoubtedly, selectively activating and transforming multiple inert chemical bonds is an even more intriguing and demanding task in synthetic chemistry. However, due to its inherent complexity and extreme challenges, this endeavour is rarely accomplished. We report a copper-mediated complete cleavage and selective transformation of multiple inert chemical bonds of three easily available feedstocks, i.e., a sp2C—H bond in indoles, three sp3C—H bonds and one C—N bond in a methyl carbon atom in TMEDA, and the C≡N triple bond in CH3CN. This reaction proceeds via tandem carbon and nitrogen atom transfer, and allows for the direct and efficient cyanation of indoles, presenting a simple and direct alternative for synthesizing 3-cyanoindoles.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202400062-sup-0001-supinfo.pdfPDF document, 6 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For reviews on unstrained C–H, C–N, C≡N bonds activation, see: (a) Davies, H. M. L.; Du Bois, J.; Yu, J.-Q. C–H Functionalization in organic synthesis. Chem. Soc. Rev. 2011, 40, 1855–1856; (b) Yang, L.; Huang, H. Transition-Metal-Catalyzed Direct Addition of Unactivated C–H Bonds to Polar Unsaturated Bonds. Chem. Rev. 2015, 115, 3468–3517; (c) Yi, H.; Zhang, G.; Wang, H.; Huang, Z.; Wang, J.; Singh, A. K.; Lei, A. Recent Advances in Radical C–H Activation/Radical Cross- Coupling. Chem. Rev. 2017, 117, 9016–9085; (d) Ouyang, K.; Hao, W.; Zhang, W.-X.; Xi, Z. Transition-Metal-Catalyzed Cleavage of C–N Single Bonds. Chem. Rev. 2015, 115, 12045–12090; (e) Wang, Q.; Su, Y.; Li, L.; Huang, H. Transition-metal catalysed C–N bond activation. Chem. Soc. Rev. 2016, 45, 1257–1272; (f) Garcı́a-Cárceles, J.; Bahou, K. A.; Bower, J. F. Recent Methodologies That Exploit Oxidative Addition of C–N Bonds to Transition Metals. ACS Catal. 2020, 10, 12738–12759.
- 2Selected examples: (a) Wu, L.-J.; Wang, Q.; Guo, J.; Wei, J.; Chen, P.; Xi, Z. From Dinitrogen to N-Containing Organic Compounds: Using Li2CN2 as a Synthon. Angew. Chem. Int. Ed. 2023, 62, e202219298;
(b) Asakawa, H.; Lee, K.-H.; Lin, Z.; Yamashita, M. Facile Scission of Isonitrile Carbon-Nitrogen Triple Bond Using a Diborane(4) Reagent. Nat. Commun. 2014, 5, 4245–4253;
(c) Zhai, D.-D.; Zhang, S.-Q.; Xie, S.-J.; Wu, R.-K.; Liu, F.; Xi, Z.-F.; Hong, X.; Shi, Z.-J. (n-Bu)4NBr-Promoted N2 Splitting to Molybdenum Nitride. J. Am. Chem. Soc. 2022, 144, 14071–14078;
(d) Li, Z.; Liu, L.; Xu, K.; Huang, T.; Li, X.; Song, B.; Chen, T. Palladium-catalyzed N-acylation of tertiary amines by carboxylic acids: A method for the synthesis of amides. Org. Lett. 2022, 22, 5517–5521;
10.1021/acs.orglett.0c01869 Google Scholar(e) Gayyur; Choudhary, S.; Kant, R.; Ghosh, N. Nitrogen Atom Transfer Enables the (5+1) Annulation Reaction to Access Aminoisoquinolines. Org. Lett. 2023, 25, 4270–4275; (f) Liu, J.; Qiu, X.; Huang, X.; Luo, X.; Zhang, C.; Wei, J.; Pan, J.; Liang, Y.; Zhu, Y.; Qin, Q.; Song, S.; Jiao, N. From alkylarenes to anilines via site-directed carbon–carbon amination. Nat. Chem. 2019, 11, 71–77; (g) Zhu, M.; Chai, Z.; Lv, Z.-J.; Li, T.; Liu, W.; Wei, J.; Zhang, W. -X. Selective Cleavage of the Strong or Weak C–C Bonds in Biphenylene Enabled by Rare-Earth Metals. J. Am. Chem. Soc. 2023, 145, 6633–6638; (h) Tang, R.-Y.; Li, G.; Yu, J.-Q. Conformation-induced remote meta-C–H activation of amines. Nature 2014, 507, 215–220.
- 3(a) Qiu, X.; Sang, Y.; Wu, H.; Xue, X.-S.; Yan, Z.; Wang, Y.; Cheng, Z.; Wang, X.; Tan, H.; Song, S.; Zhang, G.; Zhang, X.; Houk, K. N.; Jiao, N. Cleaving arene rings for acyclic alkenylnitrile synthesis. Nature 2021, 597, 64–69; (b) He, Y.; Zheng, Z.; Liu, Y.; Qiao, J.; Zhang, X.; Fan, X. Selective Cleavage and Tunable Functionalization of the C–C/C–N Bonds of N-Arylpiperidines Promoted by tBuONO. Org. Lett. 2019, 21, 1676–1680; (c) Li, T.; Wang, Z.; Zhang, M.; Zhang, H.-J.; Wen, T.-B. Rh/Cu-catalyzed multiple C–H, C–C, and C–N bond cleavage: facile synthesis of pyrido[2,1-a]indoles from 1-(pyridin-2-yl)-1H-indoles and γ-substituted propargyl alcohols. Chem. Commun. 2015, 51, 6777–6780; (d) Liu, H.-W.; Wang, D.-L.; Jiang, N.-Q.; Li, H.-Y.; Cai, Z.-J.; Ji, S.-J. Divergent synthesis of α-functionalized amides through selective N–O/C–C or N–O/C–C/C–N cleavage of aza-cyclobutanone oxime esters. Chem. Commun. 2021, 57, 9618–9621; (e) Yu, S.; Lv, N.; Liu, Z.; Zhang, Y. Cu(II)-Mediated C−C/C−O Bond Formation via C−H/C−C Bond Cleavage: Access to Benzofurans Using Amide as a Traceless Directing Group. Adv. Synth. Catal. 2020, 362, 118–125; (f) He, Y.; Yang, J.; Zhang, X.; Fan, X. Selective cleavage and reconstruction of C–N/C–C bonds in saturated cyclic amines: tunable synthesis of lactams and functionalized acyclic amines. Org. Chem. Front. 2021, 8, 5118–5123; (g) Li, W.; Zhang, S.; Feng, X.; Yu, X.; Yamamoto, Y.; Bao, M. A strategy for amide C-N Bond activation with ruthenium catalyst: selective aromatic acylation. Org. Lett. 2021, 23, 2521–2526; (h) Wu, X.; Zhao, Y.; Ge, H. Direct Aerobic Carbonylation of C(sp2)–H and C(sp3)–H Bonds through Ni/Cu Synergistic Catalysis with DMF as the Carbonyl Source. J. Am. Chem. Soc. 2015, 137, 4924–4927; (i) Min, X.-T.; Ji, D.-W.; Zheng, H.; Chen, B.-Z.; Hu, Y.-C.; Wan, B.; Chen, Q.-A. Cobalt-Catalyzed Regioselective Carboamidation of Alkynes with Imides Enabled by Cleavage of C–N and C–C Bonds. Org. Lett. 2020, 22, 3386–3391; (j) Guo, G.; Wan, S.; Si, X.; Jiang, Q.; Jia, Y.; Yang, L.; Zhou, W. From Simple to Complex: Rhodium(III)-Catalyzed C–C Bond Cleavage and C–H Bond Functionalization for the Synthesis of 3a,8b-Dihydro-1H-cyclopenta[b]benzofuran-1-ones. Org. Lett. 2017, 19, 5026–5029; (k) Liu, J.; Zhou, K.; Sun, S.; Gao, M.; Xu, B. Copper Nitrate-Mediated Selective Bond Cleavage of Alkynes: Diverse Synthesis of Isoxazoles. Chin. J. Chem. 2023, 41, 3299–3304.
- 4(a) Roque, J. B.; Kuroda, Y.; Göttemann, L. T.; Sarpong, R. Deconstructive diversification of cyclic amines. Nature 2018, 564, 244–248; (b) Zhu, M.-H.; Cheng, Z.; Wei, J.; Tan, H.; Jiao, N. Nitrogenation of Amides via C–C and C–N Bond Cleavage. CCS Chem. 2023, 5, 1061–1068; (c) Jurczyk, J.; Woo, J.; Kim, S. F.; Dherange, B. D.; Sarpong, R.; Levin, M. D. Single-atom logic for heterocycle editing. Nat. Synth. 2022, 1, 352–364.
- 5(a) Su, L.; Ren, T.; Dong, J.; Liu, L.; Xie, S.; Yuan, L.; Zhou, Y.; Yin, S.-F. Cu(I)-Catalyzed 6-endo-dig Cyclization of Terminal Alkynes, 2-Bromoaryl Ketones, and Amides toward 1-Naphthylamines: Applications and Photophysical Properties. J. Am. Chem. Soc. 2019, 141, 2535–2544; (b) He, J.; Dong, J.; Su, L.; Wu, S.; Liu, L.; Yin, S.-F.; Zhou, Y. Selective oxidative cleavage of 3-methylindoles with primary amines affording quinazolinones. Org. Lett. 2020, 22, 2522–2526; (c) Su, L.; Xie, S.; Dong, J.; Liu, F.; Yin, S.-F.; Zhou, Y. Copper-Catalyzed Nitrogen Atom Transfer to Isoquinolines via C–N Triple Bond Cleavage and Three-Component Cyclization. Org. Lett. 2022, 24, 5994–5999; (d) Liu, L.; Dong, J.; Zhang, Y.; Zhou, Y.; Yin, S.-F. Cu-mediated nitrogen atom transfer via C≡N bond cleavage. Org. Biomol. Chem. 2015, 13, 9948–9952.
- 6(a) Wu, Q.; Luo, Y.; Lei, A.; You, J. Aerobic Copper-Promoted Radical-Type Cleavage of Coordinated Cyanide Anion: Nitrogen Transfer to Aldehydes to Form Nitriles. J. Am. Chem. Soc. 2016, 138, 2885–2888; (b) Liu, W.; Tang, P.; Zheng, Y.; Ren, Y.-L.; Tian, X.; An, W.; Zheng, X.; Guo, Y.; Shen, Z. Cu2O Catalyzed Conversion of Benzyl Alcohols into Aromatic Nitriles via the Complete Cleavage of the C≡N Triple Bond in the Cyanide Anion. Chem. Asian. J. 2021, 16, 3509−3513; (c) Wang, C.; Geng, X.; Zhao, P.; Zhou, Y.; Wu, Y.-D.; Wu, A.-X. Employing thiocyanate salts as a nitrogen source via C≡N bond cleavage: divergent synthesis of α-ketoamides and 2-acyloxazoles. Org. Chem. Front. 2019, 6, 2534–2538.
- 7(a) Larock, R. C. Comprehensive Organic Transformations: A Guide to Functional Group Preparations, Wiley-VCH, New York, 1989;
(b) Rappoport, Z. Chemistry of the Cyano Group, John Wiley & Sons, London, 1970.
10.1002/9780470771242 Google Scholar
- 8Recent examples: (a) Zhou, Y.; Zhou, L.; Jesikiewicz, L. T.; Liu, P.; Buchwald, S. L. Synthesis of Pyrroles through the CuH-Catalyzed Coupling of Enynes and Nitriles. J. Am. Chem. Soc. 2020, 142, 9908–9914; (b) Huang, H.-G.; Zheng, Y.-Q.; Zhong, D.; Deng, J.-L.; Liu, W.-B. Reductive Aza-Pauson-Khand Reaction of Nitriles. J. Am. Chem. Soc. 2023, 145, 10463–10469; (c) Yao, S.; Zhou, K.; Wang, J.; Cao, H.; Yu, L.; Wu, J.; Qiu, P.; Xu, Q. Synthesis of 2-substituted quinazolines by CsOH-mediated direct aerobic oxidative cyclocondensation of 2-aminoarylmethanols with nitriles in air. Green Chem. 2017, 19, 2945–2951; (d) Ma, P.; Wang, Y.; Wang, J.; Ma, N. LiN (SiMe3)2/KOtBu- Promoted Synthesis of Isoquinolone Derivatives from 2-Methylaryl Aldehydes and Nitriles. J. Org. Chem. 2023, 88, 7425–7430; (e) Yu, X.; Gao, L.; Jia, L.; Yamamoto, Y.; Bao, M. Synthesis of Quinazolin-4(3H)- ones via the Reaction of 2-Halobenzamides with Nitriles. J. Org. Chem. 2018, 83, 10352–10358; (f) Wang, A.; Lv, P.; Liu, Y. 4,5-Dihydro-1,2,4-oxadiazole as a Single Nitrogen Transfer Reagent: Synthesis of Functionalized Isoxazoles Assisted by Sc(OTf)3 or Au(I)/Sc(OTf)3 Synergistic Catalysis. Org. Lett. 2023, 25, 4377–4382; (g) Cao, J.; Lv, D.; Yu, F.; Chiou, M.-F.; Li, Y.; Bao, H. Regioselective Three-Component Synthesis of Vicinal Diamines via 1, 2-Diamination of Styrenes. Org. Lett. 2021, 23, 3184–3189.
- 9For reviews, see: (a) Humphrey, G. R.; Kuethe, J. T. Practical Methodologies for the Synthesis of Indoles. Chem. Rev. 2006, 106, 2875–2911; (b) Kochanowska-Karamyan, A. J.; Hamann, M. T. Marine Indole Alkaloids: Potential New Drug Leads for the Control of Depression and Anxiety. Chem. Rev. 2010, 110, 4489–4497.
- 10(a) Ding, S.; Jiao, N. Direct Transformation of N,N-Dimethylformamide to −CN: Pd-Catalyzed Cyanation of Heteroarenes via C–H Functionalization. J. Am. Chem. Soc. 2011, 133, 12374–12377; (b) Zhao, D.; Xu, P.; Ritter, T. Palladium-Catalyzed Late-Stage Direct Arene Cyanation. Chem 2019, 5, 97–107; (c) Nagase, Y.; Sugiyama, T.; Nomiyama, S.; Yonekura, K.; Tsuchimoto, T. Zinc-Catalyzed Direct Cyanation of Indoles and Pyrroles: Nitromethane as a Source of a Cyano Group. Adv. Synth. Catal. 2014, 356, 347–352; (d) Xiao, J.; Li, Q.; Chen, T.; Han, L.-B. Copper-mediated selective aerobic oxidative C3-cyanation of indoles with DMF. Tetrahedron Lett. 2015, 56, 5937–5940; (e) Zhao, M.; Zhang, W.; Shen, Z. Cu-Catalyzed Cyanation of Indoles with Acetonitrile as a Cyano Source. J. Org. Chem. 2015, 80, 8868–8873; (f) Wang, L.; Shao, Y.; Cheng, J. Application of combined cyanide sources in cyanation reactions. Org. Biomol. Chem. 2021, 19, 8646–8655; (g) Liu, B.; Liu, M.; Li, Q.; Li, Y.; Feng, K.; Zhou, Y. The palladium-catalyzed direct C3-cyanation of indoles using acetonitrile as the cyanide source. Org. Biomol. Chem. 2020, 18, 6108–6114; (h) Zhang, G.; Ren, X.; Chen, J.; Hu, M.; Cheng, J. Copper-Mediated Cyanation of Aryl Halide with the Combined Cyanide Source. Org. Lett. 2011, 13, 5004–5007.
- 11(a) Ren, X.; Chen, J.; Chen, F.; Cheng, J. The palladium-catalyzed cyanation of indole C–H bonds with the combination of NH4HCO3 and DMSO as a safe cyanide source. Chem. Commun. 2011, 47, 6725–6727; (b) Liu, B.; Wang, J.; Zhang, B.; Sun, Y.; Wang, L.; Chen, J.; Cheng, J. Copper-mediated C3-cyanation of indoles by the combination of amine and ammonium. Chem. Commun. 2014, 50, 2315–2317; (c) Kim, J.; Kim, H.; Chang, S. Copper-Mediated Selective Cyanation of Indoles and 2-Phenylpyridines with Ammonium Iodide and DMF. Org. Lett. 2012, 14, 3924−3927.
- 12(a) Li, X.; Gu, X.; Li, Y.; Li, P. Aerobic Transition-Metal-Free Visible-Light Photoredox Indole C-3 Formylation Reaction. ACS Catal. 2014, 4, 1897–1900; (b) Zhang, L.; Peng, C.; Zhao, D.; Wang, Y.; Fu, H.-J.; Shen, Q.; Li, J.-X. Cu(II)-catalyzed C–H (sp3) oxidation and C–N cleavage: base-switched methylenation and formylation using tetramethylethylenediamine as a carbon source. Chem. Commun. 2012, 48, 5928–5930; (c) Zhao, D.; Wang, Y.; Zhu, M.-X.; Shen, Q.; Zhang, L.; Du, Y.; Li, J.-X. Copper(II)-catalyzed C–H (sp3) oxidation and C–N cleavage: synthesis of methylene-bridged compounds using TMEDA as a carbon source in water. RSC Adv. 2013, 3, 10272–10276; (d) Chen, J.; Liu, B.; Liu, D.; Liu, S.; Cheng, J. The Copper-Catalyzed C-3-Formylation of Indole C−H Bonds using Tertiary Amines and Molecular Oxygen. Adv. Synth. Catal. 2012, 354, 2438−2442.
- 13(a) Surry, D.; Buchwald, S. Diamine ligands in copper-catalyzed reactions. Chem. Sci. 2010, 1, 13–31; (b) Collman, J.; Zhong, M. An Efficient Diamine·Copper Complex-Catalyzed Coupling of Arylboronic Acids with Imidazoles. Org. Lett. 2000, 2, 1233–1236.
- 14(a) Murahashi, S.-I.; Komiya, N.; Terai, H.; Nakae, T. Aerobic Ruthenium-Catalyzed Oxidative Cyanation of Tertiary Amines with Sodium Cyanide. J. Am. Chem. Soc. 2003, 125, 15312–15313; (b) Dowers, T.; Rock, D.; Rock, D.; Jones, J. Kinetic Isotope Effects Implicate the Iron−Oxene as the Sole Oxidant in P450-Catalyzed N-Dealkylation. J. Am. Chem. Soc. 2004, 126, 8868–8869.
- 15 Wu, W.; Su, W. Mild and Selective Ru-Catalyzed Formylation and Fe-Catalyzed Acylation of Free (N–H) Indoles Using Anilines as the Carbonyl Source. J. Am. Chem. Soc. 2011, 133, 11924–11927.




