Controllable Construction of Vinyl Sulfones and β-Keto Selenosulfones via Selective Oxidative Sulfonylation of Alkenes
Corresponding Author
Xiang Liu
School of Chemistry and Chemical Engineering, Guangdong Pharmaceutical University, Zhongshan, Guangdong, 528458 China
*E-mail: [email protected], [email protected]Search for more papers by this authorYuan Zhang
School of Chemistry and Chemical Engineering, Guangdong Pharmaceutical University, Zhongshan, Guangdong, 528458 China
Search for more papers by this authorYi Zheng
School of Chemistry and Chemical Engineering, Guangdong Pharmaceutical University, Zhongshan, Guangdong, 528458 China
Search for more papers by this authorChangfeng Huang
School of Chemistry and Chemical Engineering, Guangdong Pharmaceutical University, Zhongshan, Guangdong, 528458 China
Search for more papers by this authorCorresponding Author
Hua Cao
School of Chemistry and Chemical Engineering, Guangdong Pharmaceutical University, Zhongshan, Guangdong, 528458 China
*E-mail: [email protected], [email protected]Search for more papers by this authorCorresponding Author
Xiang Liu
School of Chemistry and Chemical Engineering, Guangdong Pharmaceutical University, Zhongshan, Guangdong, 528458 China
*E-mail: [email protected], [email protected]Search for more papers by this authorYuan Zhang
School of Chemistry and Chemical Engineering, Guangdong Pharmaceutical University, Zhongshan, Guangdong, 528458 China
Search for more papers by this authorYi Zheng
School of Chemistry and Chemical Engineering, Guangdong Pharmaceutical University, Zhongshan, Guangdong, 528458 China
Search for more papers by this authorChangfeng Huang
School of Chemistry and Chemical Engineering, Guangdong Pharmaceutical University, Zhongshan, Guangdong, 528458 China
Search for more papers by this authorCorresponding Author
Hua Cao
School of Chemistry and Chemical Engineering, Guangdong Pharmaceutical University, Zhongshan, Guangdong, 528458 China
*E-mail: [email protected], [email protected]Search for more papers by this authorComprehensive Summary
The selective oxidative sulfonylation of alkenes with selenium sulfonate depended on the reaction conditions. The electrochemical C—H sulfonylation proceeded smoothly to afford (E)-vinyl sulfones with good selectivity in an undivided cell without external oxidant. While aerobic trifunctionalization of alkenes occurred in the presence of KI in the air, which provides β-keto selenosulfones via the formation of C—O, C—S, and C—Se bonds in one-pot. Following control experiments, a plausible mechanism is proposed to rationalize the experimental results.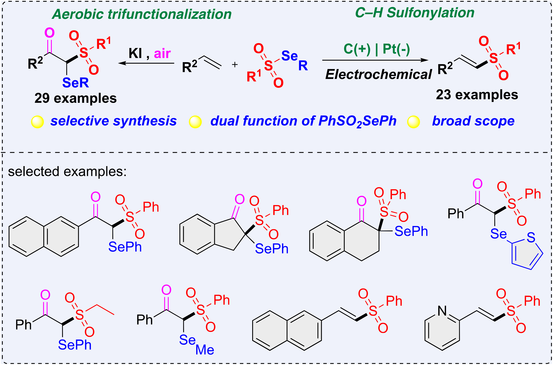
Supporting Information
| Filename | Description |
|---|---|
| cjoc202400026-sup-0001-supinfo.pdfPDF document, 9.3 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For selected reviews, see: (a) Dong, Z.; Ren, Z.; Thompson, S. J.; Xu, Y.; Dong, G. Transition-Metal-Catalyzed C−H Alkylation Using Alkenes. Chem. Rev. 2017, 117, 9333−9403; (b) Jensen, K. H.; Sigman, M. S. Mechanistic Approaches to Palladium-Catalyzed Alkene Difunctionalization Reactions. Org. Biomol. Chem. 2008, 6, 4083−4088; (c) McDonald, R. I.; Liu, G.; Stahl, S. S. Palladium(II)-Catalyzed Alkene Functionalization via Nucleopalladation: Stereochemical Pathways and Enantioselective Catalytic Applications. Chem. Rev. 2011, 111, 2981−3019.
- 2 Lee, M.; Ikejiri, M.; Klimpel, D.; Toth, M.; Espahbodi, M.; Hesek, D.; Forbes, C.; Kumarasiri, M.; Noll, B. C.; Chang, M.; Mobashery, S. Structure−Activity Relationship for Thiirane-Based Gelatinase Inhibitors. ACS Med. Chem. Lett. 2012, 3, 490−495.
- 3(a) Eto, H.; Kaneko, Y.; Takeda, S.; Tokizawa, M.; Sato, S.; Yoshida, K.; Namiki, S.; Ogawa, M.; Maebashi, K.; Ishida, K.; Matsumoto, M.; Asaoka, T. New Antifungal 1,2,4-Triazoles with Difluoro(Substituted Sulfonyl)Methyl Moiety. Chem. Pharm. Bull. 2001, 49, 173−182; (b) Fortugno, C.; Varchi, G.; Guerrini, A.; Carrupt, P.-A.; Bertucci, C. Optical Biosensor Analysis in Studying New Synthesized Bicalutamide Analogs Binding to Androgen Receptor. J. Pharm. Biomed. Anal. 2014, 95, 151−157; (c) Shen, Y.; Zificsak, C. A.; Shea, J. E.; Lao, X.; Bollt, O.; Li, X.; Lisko, J. G.; Theroff, J. P.; Scaife, C. L.; Ator, M. A.; Ruggeri, B. A.; Dorsey, B. D.; Kuwada, S. K. Design, Synthesis, and Biological Evaluation of Sulfonyl Acrylonitriles as Novel Inhibitors of Cancer Metastasis and Spread. J. Med. Chem. 2015, 58, 1140−1158.
- 4(a) Ashcroft, C. P.; Hellier, P.; Pettman, A.; Watkinson, S. Second Generation Process Research towards Eletriptan: A Fischer Indole Approach. Org. Process Res. Dev. 2011, 15, 98−103; (b) Madasu, S. B.; Vekariya, N. A.; Kiran, M. N.; Gupta, B.; Islam, A.; Douglas, P. S.; Babu, K. R. Synthesis of Compounds Related to the Anti-Migraine Drug Eletriptan Hydrobromide. Beilstein J. Org. Chem. 2012, 8, 1400−1405.
- 5(a) Julia, M.; Paris, J. M. Syntheses Using Sulfones. 5. Method for General Synthesis of Doubles, Tetrahedron Lett. 1973, 14, 4833–4836; (b) Blakemore, P. R. The Modified Julia Olefination: Alkene Synthesis via the Condensation of Metallated Heteroarylalkylsulfones with Carbonyl Compounds. J. Chem. Soc., Perkin Trans. 1 2002, 2563−2585; (c) Song, Q.; Zhao, H.; Sun, Y.; Jiang, H.; Zhang, M. Direct C(sp3)–H Sulfonylation of Xanthene Derivatives with Sodium Sulfinates by Oxidative Copper Catalysis. Chin. J. Chem. 2022, 40, 371–377; (d) Song, R.; Xie, Y. Recent Advances in Oxidative ipso-Annulation of N-Arylpropiolamides. Chin. J. Chem. 2017, 35, 280–288.
- 6(a) Liu, W.; Hao, L.; Zhang, J.; Zhu, T. Progress in the Electrochemical Reactions of Sulfonyl Compounds. ChemSusChem 2022, 15, e20210255; (b) Reddy, R. J.; Kumari, A. H.; Kumar, J. J. Recent advances in the synthesis and applications of β-keto sulfones: new prospects for the synthesis of β-keto thiosulfones. Org. Biomol. Chem. 2021, 19, 3087–3118; (c) Mampuys, P.; McElroy, C. R.; Clark, J. H.; Orru, R. V. A.; Maes, B. U. W. Thiosulfonates as Emerging Reactants: Synthesis and Applications, Adv. Synth. Catal. 2020, 362, 3–64; (d) Pannecoucke, X.; Besset, T. Use of ArSO2SRf Reagents: An Efficient Tool for the Introduction of SRf Moieties. Org. Biomol. Chem. 2019, 17, 1683–1693.
- 7(a) Shen, Z.-J.; Huang, B.; Ma, N.; Yao, L.; Yang, C.; Guo, L.; Xia, W. Transition Metal-Free Synthesis of Sulfonyl- and Bromo-Substituted Indolo[2,1-α]isoquinoline Derivatives through Electrochemical Radical Cascade Cyclization. Adv. Synth. Catal. 2021, 363, 1944–1954;
(b) Fang, Y.; Xu, D.; Yu, Y.; Tang, R.; Dai, S.; Wang, Z.; Zhang, W. Controlled Synthesis of β-Keto Sulfones and Vinyl Sulfones under Electrochemical Oxidation. Eur. J. Org. Chem. 2022, 13, e202200091;
10.1002/ejoc.202200091 Google Scholar(c) Bi, W.-Z.; Zhang, W.-J.; Feng, S.-X.; Li, Z.-J.; Ma, H.-L.; Zhu, S.-H.; Chen, X.-L.; Qu, L.-B.; Zhao, Y.-F. Temperature-dependent synthesis of vinyl sulfones and β-hydroxy sulfones from t-butylsulfinamide and alkenes under aerobic conditions. New J. Chem. 2019, 43, 17941–17945; (d) Zhou, X.; Peng, Z.; Wang, P. G.; Liu, Q.; Jia, T. Atom Transfer Radical Addition to Styrenes with Thiosulfonates Enabled by Synergetic Copper/Photoredox Catalysis. Org. Lett. 2021, 23, 1054–1059; (e) Xia, S.; Wu, L.; Zhai, G.; Wang, Z.; Wu, J. Visible-light- promoted CO2 oxidative 1,2-thiosulfonylation of styrenes with sodium sulfinates and thiophenols. Org. Chem. Front. 2023, 10, 4002–4009; (f) Li, H.; Shan, C.; Tung, C. H.; Xu, Z. Dual Gold and Photoredox Catalysis: Visible Light-Mediated Intermolecular Atom Transfer Thiosulfonylation of Alkenes. Chem. Sci. 2017, 8, 2610–2615; (g) Gadde, K.; Mampuys, P.; Guidetti, A.; Ching, H. Y. V.; Herrebout, W. A.; Van Doorslaer, S.; Abbaspour Tehrani, K.; Maes, B. U. W. Thiosulfonylation of Unactivated Alkenes with Visible-Light Organic Photocatalysis. ACS Catal. 2020, 10, 8765–8779; (h) Liang, Q.; Walsh, P. J.; Jia, T. Copper-Catalyzed Intermolecular Difunctionalization of Styrenes with Thiosulfonates and Arylboronic Acids via a Radical Relay Pathway. ACS Catal. 2020, 10, 2633−2639.
- 8(a) Liu, J.; Wu, S.; Yu, J.; Lu, C.; Wu, Z.; Wu, X.; Xue, X.; Zhu, C. Polarity Umpolung Strategy for the Radical Alkylation of Alkenes. Angew. Chem. Int. Ed. 2020, 59, 8195–8202; (b) Huang, S.; Thirupathi, N.; Tung, C.-H.; Xu, Z. Copper-Catalyzed Oxidative Trifunctionalization of Olefins: An Access to Functionalized β-Keto Thiosulfones. J. Org. Chem. 2018, 83, 9449-9455; (c) Huang, S.; Li, H.; Xie, T.; Wei, F.; Tung, C.-H.; Xu, Z. Scandium-Catalyzed Electrophilic Alkene Difunctionalization: Regioselective Synthesis of Thiosulfone Derivatives. Org. Chem. Front. 2019, 6, 1663–1666; (d) Li, W.; Zhou, L. Visible-light-driven radical 1,3-addition of selenosulfonates to vinyldiazo compounds. Green Chem. 2021, 23, 6652–6658.
- 9(a) Wang, F.; Liu, B.-X.; Rao, W.; Wang, S.-Y. Metal-Free Chemoselective Reaction of Sulfoxonium Ylides and Thiosulfonates: Diverse Synthesis of 1,4-Diketones, Aryl Sulfursulfoxonium Ylides, and β-Keto Thiosulfones Derivatives. Org. Lett. 2020, 22, 6600–6604; (b) Tan, Z.; Chen, F.; Huang, G.; Li, Y.; Jiang, H.; Zeng, W. Photocatalyzed Thiosulfonylation of Sila-enynes with Thiosulfonates. Org. Lett. 2023, 25, 2846–2851; (c) Qian, P.; Bi, M.; Su, J.; Zha, Z.; Wang, Z. Electrosynthesis of (E)-Vinyl Sulfones Directly from Cinnamic Acids and Sodium Sulfinates via Decarboxylative Sulfono Functionalization. J. Org. Chem. 2016, 81, 4876–4882; (d) Lai, Y.-L.; Mo, Y.; Yan, S.; Zhang, S.; Zhu, L.; Luo, J.; Guo, H.; Cai, J.; Liao, J. Electrochemical sulfonylation of alkenes with sulfonyl hydrazides: a metal- and oxidant-free protocol for the synthesis of (E)-vinyl sulfones in water. RSC Adv. 2020, 10, 33155–33160.
- 10(a) Yuan, Y.; Lei, A. Is electrosynthesis always green and advantageous compared to traditional methods? Nat. Commun. 2020, 11, 802–805;
(b) Yan, M.; Kawamata, Y.; Baran, P. S. Synthetic Organic Electrochemical Methods Since 2000: On the Verge of a Renaissance. Chem. Rev. 2017, 117, 13230–13319;
(c) Francke, R.; Little, R. D. Redox catalysis in organic electrosynthesis: basic principles and recent developments. Chem. Soc. Rev. 2014, 43, 2492–2521;
(d) Zhang, X.; Xu, S.; Yang, X.; Pang, W. KI-Catalyzed Allylic Sulfonation of α-Methylstyrene Derivatives with Sulfonylhydrazides via Electrochemistry. J. Org. Chem. 2023, 88, 7483–7488;
(e) Zhou, X.-Q.; Tang, H.-T.; Cui, F.-H.; Liang, Y.; Li, S.-H.; Pan, Y.-M. Electrocatalytic three- component reactions: synthesis of tellurium containing oxazolidinone for anticancer agents. Green Chem. 2023, 25, 5024−5029;
(f) Tang, H.-T.; Pan, Y.-Z.; Pan, Y.-M. Research progress in electrochemical/photochemical utilization of methanol as a C1 source. Green Chem. 2023, 25, 8313−8327;
(g) Xiong, T.-K.; Xia, Q.; Zhou, X.-Q.; Li, S.-H.; Cui, F.-H.; Tang, H.-T.; Pan, Y.-M.; Liang, Y. Electrochemically mediated fixation of CO2: synthesis of functionalized oxazolidine-2,4- diones by three-component reactions. Adv. Synth. Catal. 2023, 365, 2183−2187;
(h) Luo, Y.-C.; Pan, X.-J.; Yuan, G.-Q. An efficient electrochemical synthesis of vinyl sulfones from sodium sulfinates and olefins. Tetrahedron 2015, 17, 2119–2123;
10.1016/j.tet.2015.02.048 Google Scholar(i) Xu, Z.; Yao, J.; Zhong, K.; Lin, S.; Hu, X.; Ruan, Z. Electrochemical Selenylation of Sulfoxonium Ylides for the Synthesis of gem-Diselenides as Antimicrobials against Fungi. J. Org. Chem. 2023, 88, 5572–5585; (j) Hasimujiang, B.; Ruan, Z. Electrochemical Synthesis of Organoselenium Compounds: A Graphical Review. SynOpen 2023, 7, 511–520.
- 11(a) Huang, C.; Hu, J.; Chen, G.; Wu, M.; Cao, H.; Liu, X. Electrochemical Oxidative Cyclization of Alkenes, Boronic Acids, and Dichalcogenides to Access Chalcogenated Boronic Esters and 1,3-Diols. Org. Chem. Front. 2022, 9, 12–18; (b) Liu, X.; Jiang, W.; Huang, C.; Ma, S.; Wang, Q.; Cao, H. Electrochemical phosphorothiolation and 1,4-S→C phospho-Fries rearrangement: controlled access to phosphorothiolated and mercapto-phosphono substituted indolizines. Org. Chem. Front. 2023, 10, 5198–5204; (c) Liu, X.; Wang, Y.; Song, D.; Wang, Y.; Cao, H. Electrochemical regioselective selenylation/oxidation of N-alkylisoquinolinium salts via double C(sp2)–H bond functionalization. Chem. Commun. 2020, 56, 15325–15328.
- 12(a) Liu, X.; Lin, J.; Zhuang, C.; Zhong, J.; Song, D.; Zhao, J.; Cao, H. Switchable hydroxysulfonyloxylation and defluorination−decarboxylation sulfonylation of gem-difluoroalkenes with sodium sulfinate via aerobic oxidation. Org. Chem. Front. 2021, 8, 6220−6225; (b) Zhou, J.; Li, W.; Zheng, H.; Pei, Y.; Liu, X.; Cao, H. Visible Light-Induced Cascade Cyclization of 3-Aminoindazoles, Ynals, and Chalcogens: Access to Chalcogen-Containing Pyrimido[1,2-b]-indazoles. Org. Lett. 2021, 23, 2754–2759; (c) Liu, X.; Song, D.; Zhang, Z.; Lin, J.; Zhuang, C.; Zhan, H.; Cao, H. Regioselective C-H dithiocarbamation of indolizines with tetraalkylthiuram disulfide under metal-free conditions. Org. Biomol. Chem. 2021, 19, 5284–5288.




