Stereospecific Assembly of Trisubstituted Alkenes via Photoinduced Nitrogen-Centered Radical-Triggered C—C Bond Cleavage/Functionalization of Oxime Esters
Yu Bao
School of Pharmacy, Changzhou University, Changzhou, Jiangsu, 213164 China
Search for more papers by this authorZhi-Jie Song
School of Pharmacy, Changzhou University, Changzhou, Jiangsu, 213164 China
Search for more papers by this authorJin-Long Dai
School of Pharmacy, Changzhou University, Changzhou, Jiangsu, 213164 China
Search for more papers by this authorShenghu Yan
School of Pharmacy, Changzhou University, Changzhou, Jiangsu, 213164 China
Search for more papers by this authorCorresponding Author
Yue Zhang
School of Pharmacy, Changzhou University, Changzhou, Jiangsu, 213164 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Jia-Yin Wang
School of Pharmacy, Changzhou University, Changzhou, Jiangsu, 213164 China
E-mail: [email protected]; [email protected]Search for more papers by this authorGuigen Li
Department of Chemistry and Biochemistry, Texas Tech University, 79409 USA
Search for more papers by this authorYu Bao
School of Pharmacy, Changzhou University, Changzhou, Jiangsu, 213164 China
Search for more papers by this authorZhi-Jie Song
School of Pharmacy, Changzhou University, Changzhou, Jiangsu, 213164 China
Search for more papers by this authorJin-Long Dai
School of Pharmacy, Changzhou University, Changzhou, Jiangsu, 213164 China
Search for more papers by this authorShenghu Yan
School of Pharmacy, Changzhou University, Changzhou, Jiangsu, 213164 China
Search for more papers by this authorCorresponding Author
Yue Zhang
School of Pharmacy, Changzhou University, Changzhou, Jiangsu, 213164 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Jia-Yin Wang
School of Pharmacy, Changzhou University, Changzhou, Jiangsu, 213164 China
E-mail: [email protected]; [email protected]Search for more papers by this authorGuigen Li
Department of Chemistry and Biochemistry, Texas Tech University, 79409 USA
Search for more papers by this authorComprehensive Summary
A general and convenient photoredox-catalyzed acylation and alkylcyanation of MBH acetates has been established, enabling the assembly of the C(sp2)–C(sp3) bond by a nitrogen-centered radical strategy for the synthesis of trisubstituted alkenes in moderate to excellent chemical yields (48 examples in total). The reaction of MBH acetates with acyl (indanone) oxime esters afforded trisubstituted alkenes containing 1,4-dicarbonyl groups. Interestingly, the use of Eosin Y as a photocatalyst in the catalytic system resulted in the formation of distal cyano group-anchored trisubstituted alkenes via deconstructive functionalization of cycloketone oxime esters. Notably, these resulting 1,4-dicarbonyl compounds could be applied to late-stage transformations, providing important methods for the synthesis of dihydropyridazin-3(2H)-one.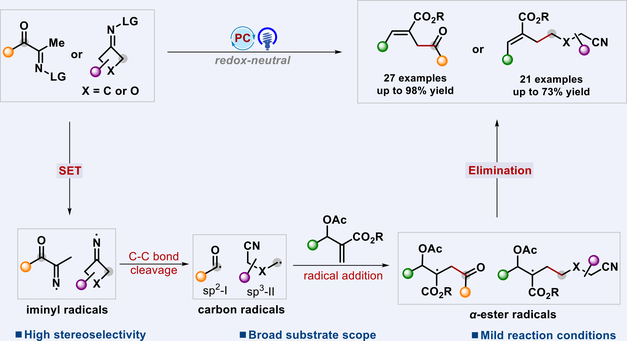
Supporting Information
| Filename | Description |
|---|---|
| cjoc202300774-sup-0001-Supinfo.pdfPDF document, 9.1 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Harper, M. J. K.; Walpole, A. L. Contrasting Endocrine Activities of cis and trans Isomers in a Series of Substituted Triphenylethylenes. Nature 1966, 212, 87; (b) Stewart, J.; Charest, M.-P.; Herr, F. A. Pharmacological investigation of potential antidepressants of the amitriptyline-type. J. Med. Chem. 1963, 6, 338–339; (c) Wang, H.; Toh, R. W.; Shi, X.; Wang, T.; Cong, X.; Wu, J. Photo-mediated selective deconstructive geminal dihalogenation of trisubstituted alkenes. Nat. Commun. 2020, 11, 4462.
- 2For selected examples, see: (a) Zhao, Q.; Hao, W.-J.; Shi, H.-N.; Xu, T.; Tu, S.-J.; Jiang, B. Photocatalytic Annulation–Alkynyl Migration Strategy for Multiple Functionalization of Dual Unactivated Alkenes. Org. Lett. 2019, 21, 9784–9789; (b) Wei, W.; Cui, H.; Yue, H.; Yang, D. Visible-light-enabled oxyazidation of alkenes leading to α-azidoketones in air. Green Chem. 2018, 20, 3197–3202; (c) Sun, K.; Wang, S.; Feng, R.; Zhang, Y.; Wang, X.; Zhang, Z.; Zhang, B. Copper-Catalyzed Radical Selenodifluoromethylation of Alkenes: Access to CF2-Containing γ-Lactams. Org. Lett. 2019, 21, 2052–2055; (d) Sarkar, S.; Banerjee, A.; Yao, W.; Patterson, E. V.; Ngai, M.-Y. Photocatalytic Radical Aroylation of Unactivated Alkenes: Pathway to β-Functionalized 1,4-, 1,6-, and 1,7-Diketones. ACS Catal. 2019, 9, 10358–10364; (e) Ma, X.; Zhang, X.; Awad, J. M.; Xie, G.; Qiu, W.; Zhang, W. One-pot synthesis of tetrahydro-pyrrolobenzodiazepines and tetrahydro-pyrrolobenzodiazepinones through sequential 1,3-dipolar cycloaddition/ N-alkylation (N-acylation)/Staudinger/aza-Wittig reactions. Green Chem. 2019, 21, 4489–4494.
- 3(a) Scott, W. J.; McMurry, J. E. Olefin synthesis via organometallic coupling reactions of enol triflates. Acc. Chem. Res. 1988, 21, 47–54; (b) Zhang, Y.; Wang, J. Alkene Synthesis Through Transition Metal- Catalyzed Cross-Coupling of N-Tosylhydrazones. Top. Curr. Chem. 2012, 327, 239–269; (c) Gu, Y.; Tian, S.-K. Olefination Reactions of Phosphorus-Stabilized Carbon Nucleophiles. Top. Curr. Chem. 2012, 327, 197–238.
- 4(a) Flynn, A. B.; Ogilvie, W. W. Stereocontrolled Synthesis of Tetrasubstituted Olefins. Chem. Rev. 2007, 107, 4698–4745;
(b) Negishi, E.-I.; Huang, Z.; Wang, G.; Mohan, S.; Wang, C.; Hattori, H. Recent Advances in Efficient and Selective Synthesis of Di-, Tri-, and Tetrasubstituted Alkenes via Pd-Catalyzed Alkenylation−Carbonyl Olefination Synergy. Acc. Chem. Res. 2008, 41, 1474–1485;
(c) Chemler, S. R.; Trauner, D.; Danishefsky, S. J. The B-Alkyl Suzuki–Miyaura Cross- Coupling Reaction: Development, Mechanistic Study, and Applications in Natural Product Synthesis. Angew. Chem. Int. Ed. 2001, 40, 4544–4568;
10.1002/1521-3773(20011217)40:24<4544::AID-ANIE4544>3.0.CO;2-N CAS PubMed Web of Science® Google Scholar(d) Wang, B.; Ong, D. Y.; Li, Y.; Pang, J. H.; Watanabe, K.; Takita, R.; Chiba, S. Stereo-controlled anti-hydromagnesiation of aryl alkynes by magnesium hydrides. Chem. Sci. 2020, 11, 5267–5272; (e) Wen, J.; Zhao, W.; Gao, X.; Ren, X.; Dong, C.; Wang, C.; Liu, L.; Li, J. Synthesis of [1,2,3]Triazolo-[1,5-a]quinoxalin-4(5H)-ones through Photoredox-Catalyzed [3 + 2] Cyclization Reactions with Hypervalent Iodine(III) Reagents. J. Org. Chem. 2022, 87, 4415–4423; (f) Liu, L.; Zhang, Y.; Zhao, W.; Wen, J.; Dong, C.; Hu, C.; Li, J. Photoredox- Catalyzed Cascade sp2 C–H Bond Functionalization to Construct Substituted Acridine with Diarylamine and Hypervalent Iodine(III) Reagents. Org. Lett. 2023, 25, 592–596.
- 5For conspicuous examples, see: (a) Edwards, J. T.; Merchant, R. R.; McClymont, K. S.; Knouse, K. W.; Qin, T.; Malins, L. R.; Vokits, B.; Shaw, S. A.; Bao, D. H.; Wei, F. L.; Zhou, T.; Eastgate, M. D.; Baran, P. S. Decarboxylative alkenylation. Nature 2017, 545, 213–218; (b) Negishi, E.; Wang, G.; Rao, H.; Xu, Z. Alkyne Elementometalation−Pd- Catalyzed Cross-Coupling. Toward Synthesis of All Conceivable Types of Acyclic Alkenes in High Yields, Efficiently, Selectively, Economically, and Safely: “Green” Way. J. Org. Chem. 2010, 75, 3151–3182.
- 6(a) Kortman, G. C.; Hull, K. L. Copper-Catalyzed Hydroarylation of Internal Alkynes: Highly Regio- and Diastereoselective Synthesis of 1,1-Diaryl, Trisubstituted Olefins. ACS Catal. 2017, 7, 6220–6224; (b) Fujihara, T.; Xu, T.; Semba, K.; Terao, J.; Tsuji, Y. Copper-Catalyzed Hydrocarboxylation of Alkynes Using Carbon Dioxide and Hydrosilanes. Angew. Chem. Int. Ed. 2011, 50, 523–527; (c) Semba, K.; Fujihara, T.; Terao, J.; Tsuji, Y. Copper-Catalyzed Highly Regio- and Stereoselective Directed Hydroboration of Unsymmetrical Internal Alkynes: Controlling Regioselectivity by Choice of Catalytic Species. Chem.-Eur. J. 2012, 18, 4179–4184; (d) Lu, X.-Y.; Liu, C.-C.; Jiang, R.-C.; Yan, L.-Y.; Liu, Q.-L.; Wang, Q.-Q.; Li, J.-M. Synthesis of trisubstituted alkenes by Ni-catalyzed hydroalkylation of internal alkynes with cycloketone oxime esters. Chem. Commun. 2020, 56, 14191–14194; (e) Lu, X.-Y.; Hong, M.-L.; Zhou, H.-P.; Wang, Y.; Wang, J.-Y.; Ge, X.-T. Trisubstituted olefin synthesis via Ni-catalyzed hydroalkylation of internal alkynes with non-activated alkyl halides. Chem. Commun. 2018, 54, 4417–4420; (f) Deng, H.-P.; Fan, X.-Z.; Chen, Z.-H.; Xu, Q.-H.; Wu, J. Photoinduced Nickel-Catalyzed Chemo- and Regioselective Hydroalkylation of Internal Alkynes with Ether and Amide α-Hetero C(sp3)–H Bonds. J. Am. Chem. Soc. 2017, 139, 13579–13584.
- 7(a) Jin, S.; Liu, K.; Wang, S.; Song, Q. Enantioselective Cobalt-Catalyzed Cascade Hydrosilylation and Hydroboration of Alkynes to Access Enantioenriched 1,1-Silylboryl Alkanes. J. Am. Chem. Soc. 2021, 143, 13124–13134; (b) Gao, Y.; Kim, N.; Mendoza, S. D.; Yazdani, S.; Vieira, A. F.; Liu, M.; Kendrick, A.; Grotjahn, D. B.; Bertrand, G.; Jazzar, R.; Engle, K. M. (CAAC) Copper Catalysis Enables Regioselective Three-Component Carboboration of Terminal Alkynes. ACS Catal. 2022, 12, 7243–7247; (c) Li, H.; Wang, F.; Zhu, S.; Chu, L. Selective Fluoromethyl Couplings of Alkynes via Nickel Catalysis. Angew. Chem. Int. Ed. 2022, 61, e202116725; (d) Bose, S. K.; Mao, L.; Kuehn, L.; Radius, U.; Nekvinda, J.; Santos, W. L.; Westcott, S. A.; Steel, P. G.; Marder, T. B. First-Row d-Block Element-Catalyzed Carbon–Boron Bond Formation and Related Processes. Chem. Rev. 2021, 121, 13238–13341.
- 8(a) Yan, M.; Lo, J.-C.; Edwards, J.-T.; Baran, P.-S. Radicals: Reactive Intermediates with Translational Potential. J. Am. Chem. Soc. 2016, 138, 12692–12714;
(b) Studer, A.; Curran, D. P. Catalysis of Radical Reactions: A Radical Chemistry Perspective. Angew. Chem. Int. Ed. 2016, 55, 58–102;
(c) Huang, H. M.; Garduno-Castro, M. H.; Morrill, C.; Procter, D. J. Catalytic cascade reactions by radical relay. Chem. Soc. Rev. 2019, 48, 4626–4638;
(d) Leifert, D.; Studer, A. The Persistent Radical Effect in Organic Synthesis. Angew. Chem. Int. Ed. 2020, 59, 74–108;
(e) Tong, S.; Li, K.; Ouyang, X.; Song, R.-J.; Li, J.-H. Recent advances in the radical-mediated decyanative alkylation of cyano (hetero) arene. Green Synth. Catal. 2021, 2, 145–155;
10.1016/j.gresc.2021.04.003 Google Scholar(f) Wei, B.; Qin, J.-H.; Yang, Y.-Z.; Xie, Y.-X.; Ouyang, X.-H.; Song, R.-J. Electrochemical radical C(sp3)–H arylation of xanthenes with electron-rich arenes. Org. Chem. Front. 2022, 9, 816–821; (g) Wu, Y.-C.; Xiao, Y.-T.; Yang, Y.-Z.; Song, R.-J.; Li, J.-H. Recent Advances in Silver-Mediated Radical Difunctionalization of Alkenes. ChemCatChem 2020, 12, 5312-5329; (h) Ma, X.; Zhang, Q.; Zhang, W. Remote Radical 1,3-, 1,4-, 1,5-, 1,6-and 1,7-Difunctionalization Reactions. Molecules 2023 28, 3027.
- 9(a) Sivaguru, P.; Wang, Z.; Zanoni, G.; Bi, X. Cleavage of carbon–carbon bonds by radical reactions. Chem. Soc. Rev. 2019, 48, 2615–2656; (b) Huang, M. H.; Hao, W. J.; Li, G.; Tu, S. J.; Jiang, B. Recent advances in radical transformations of internal alkynes. Chem. Commun. 2018, 54, 10791–10811; (c) Huang, M. H.; Hao, W. J.; Jiang, B. Recent Advances in Radical-Enabled Bicyclization and Annulation/1,n-Bifunctionalization Reactions. Chem. Asian J. 2018, 13, 2958–2977; (d) Narayanam, J. M. R.; Stephenson, C. R. J. Visible light photoredox catalysis: applications in organic synthesis. Chem. Soc. Rev. 2011, 40, 102–113; (e) Cheng, X.; Lei, A.; Mei, T. S.; Xu, H. C.; Xu, K.; Zeng, C. Recent applications of homogeneous catalysis in electrochemical organic synthesis. CCS Chem. 2022, 4, 1120–1152.
- 10(a) Chen, J.; Hu, X.; Lu, L.; Xiao, W.-J. Visible light photoredox-controlled reactions of N-radicals and radical ions. Chem. Soc. Rev. 2016, 45, 2044–2056; (b) Musacchio, A. J.; Nguyen, L. Q.; Beard, G. H.; Knowles, R. R. Catalytic olefin hydroamination with aminium radical cations: a photoredox method for direct C–N bond formation. J. Am. Chem. Soc. 2014, 136, 12217–12220; (c) Jiang, H.; An, X.; Tong, K.; Zheng, T.; Zhang, Y.; Yu, S. Visible-Light-Promoted Iminyl-Radical Formation from Acyl Oximes: A Unified Approach to Pyridines, Quinolines, and Phenanthridines. Angew. Chem. Int. Ed. 2015, 54, 4055–4059; (d) Hu, X.-Q.; Chen, J.-R.; Wei, Q.; Liu, F.-L.; Deng, Q.-H.; Beauchemin, A. M.; Xiao, W.-J. Photocatalytic Generation of N-Centered Hydrazonyl Radicals: A Strategy for Hydroamination of β,γ-Unsaturated Hydrazones. Angew. Chem. Int. Ed. 2014, 53, 12163–12167; (e) Davies, J.; Booth, S. G.; Essafi, S.; Dryfe, R. A. W.; Leonori, D. Visible-light-mediated generation of nitrogen-centered radicals: metal-free hydroimination and iminohydroxylation cyclization reactions. Angew. Chem. Int. Ed. 2015, 54, 14017–14021; (f) Zard, S. Z. Recent progress in the generation and use of nitrogen-centred radicals. Chem. Soc. Rev. 2008, 37, 1603–1618.
- 11(a) Cheng, Y.-Y.; Lei, T.; Su, L.; Fan, X.; Chen, B.; Tung, C.-H.; Wu, L.-Z. Visible light irradiation of acyl oxime esters and styrenes efficiently constructs β-carbonyl imides by a scission and four-component reassembly process. Org. Lett. 2019, 21, 8789–8794; (b) Fan, X.; Lei, T.; Chen, B.; Tung, C.-H.; Wu, L.-Z. Photocatalytic C–C bond activation of oxime ester for acyl radical generation and application. Org. Lett. 2019, 21, 4153–4158; (c) Zheng, L.; Xia, P.-J.; Zhao, Q.-L.; Qian, Y.-E.; Jiang, W.-N.; Xiang, H.-Y.; Yang, H. Photocatalytic Hydroacylation of Alkenes by Directly Using Acyl Oximes. J. Org. Chem. 2020, 85, 11989–11996; (d) Chen, P.; Xie, J.; Chen, Z.; Xiong, B.-Q.; Liu, Y.; Yang, C.-A.; Tang, K.-W. Clusters Induced Electron Redistribution to Tune Oxygen Reduction Activity of Transition Metal Single-Atom for Metal-Air Batteries. Adv. Synth. Catal. 2021, 363, 4440–4446; (e) Yu, W.-Q.; Xie, J.; Chen, Z.; Xiong, B.-Q.; Liu, Y.; Tang, K.-W. Visible-Light- Induced Transition-Metal-Free Nitrogen-Centered Radical Strategy for the Synthesis of 2-Acylated 9H-Pyrrolo[1,2-a]Indoles. J. Org. Chem. 2021, 86, 13720–13733; (f) Zhou, Q.; Xiong, F.-T.; Chen, P.; Xiong, B.-Q.; Tang, K.-W.; Liu, Y. The visible-light-induced acylation/ cyclization of alkynoates with acyl oximes for the construction of 3-acylcoumarins. Org. Biomol. Chem. 2021, 19, 9012–9020; (g) He, B.-Q.; Gao, Y.; Wang, P.-Z.; Wu, H.; Zhou, H.-B.; Liu, X.-P.; Chen, J.-R. Dual photoredox/palladium-catalyzed C–H acylation of 2-arylpyridines with oxime esters. Synlett 2021, 32, 373–377.
- 12 Fu, Y.; Zhu, S.; Zhao, X.; Huang, S. Photoredox-catalyzed coupling of acyl oxime acetates with thiophenols to give arylthioesters in water at room temperature. Green Chem. 2022, 24, 6849–6853.
- 13 Yu, X.-Y.; Chen, J.-R.; Xiao, W.-J. Visible light-driven radical-mediated C–C bond cleavage/functionalization in organic synthesis. Chem. Rev. 2021, 121, 506–561.
- 14(a) Wang, S.-C.; Shen, Y.-T.; Zhang, T.-S.; Hao, W.-J.; Tu, S.-J.; Jiang, B. Cyclic oxime esters as deconstructive bifunctional reagents for cyanoalkyl esterification of 1, 6-enynes. J. Org. Chem. 2021, 86, 15488–15497; (b) Zuo, H.-D.; Zhu, S.-S.; Hao, W.-J.; Wang, S.-C.; Tu, S.-J.; Jiang, B. Copper-catalyzed asymmetric deconstructive alkynylation of cyclic oximes. ACS Catal. 2021, 11, 6010–6019; (c) Zhao, J.-F.; Duan, X.-H.; Gu, Y.-R.; Gao, P.; Guo, L.-N. Iron-catalyzed decarboxylative olefination of cycloketone oxime esters with α, β-unsaturated carboxylic acids via C–C bond cleavage. Org. Lett. 2018, 20, 4614–4617; (d) Deng, Y.; Zhao, C.; Zhou, Y.; Wang, H.; Li, X.; Cheng, G.-J.; Fu, J. Directing-group-based strategy enabling intermolecular Heck-type reaction of cycloketone oxime esters and unactivated alkenes. Org. Lett. 2020, 22, 3524–3530.
- 15(a) Yadav, A.; Sharma, A.; Singh, K. Visible light enabled γ-trifluoromethylation of Baylis–Hillman acetates: stereoselective synthesis of trisubstituted alkenes. Org. Chem. Front. 2019, 6, 989–993; (b) Dai, X.; Cheng, D.; Guan, B.; Mao, W.; Xu, X.; Li. X. The Coupling of Tertiary Amines with Acrylate Derivatives via Visible-Light Photoredox Catalysis. J. Org. Chem. 2014, 79, 7212–7219; (c) Zhao, H.; Ni, N.; Li, X.; Cheng, D.; Xu, X. The coupling reaction of α-silylamines with Baylis-Hillman adducts by visible light photoredox catalysis. Tetrahedron Lett. 2021, 65, 152746; (d) Lebargy, C.; Schutter, C. D. Legay, R.; Pfund, E.; Lequeux, T. Radical Allylation: E-Selective Radical Conjugate Addition–Elimination Reaction from Morita–Baylis–Hillman Adducts. Synlett 2018, 29, 46–50; (e) Mandal, S. K., Paira, M.; Roy, S. C. Titanocene(III) Chloride Mediated Radical-Induced Addition to Baylis−Hillman Adducts: Synthesis of (E)- and (Z)-Trisubstituted Alkenes and α-Methylene/Arylidene δ-Lactones. J. Org. Chem. 2008, 73, 3823–3827; (f) Ye, H.; Zhao, H.; Ren, S.; Ye, H.; Cheng, D.; Li, X.; Xu, X. The coupling of alkylboronic acids and esters with Baylis–Hillman derivatives by Lewis base/photoredox dual catalysis. Tetrahedron Lett. 2019, 60, 1302–1305; (g) Bertuzzi, G.; Ombrosi, G.; Bandini, M. Regio- and Stereoselective Electrochemical Alkylation of Morita–Baylis–Hillman Adducts. Org. Lett. 2022, 24, 4354–4359.
- 16(a) Wang, J.-Y.; Zhang, S.; Tang, Y.; Yan, S.; Li, G. Copper-Catalyzed Annulation–Trifluoromethyl Functionalization of Enynones. Org. Lett. 2023, 25, 2509–2514; (b) Wang, J.-Y.; Li, G.; Hao, W.-J.; Jiang, B. Catalytic Benzannulation Reactions of Enynones for Accessing Heterocycle-Incorporating Diarylmethanes. Synlett 2023, 34, 243–248; (c) Wang, J.-Y.; Zhang, S.; Yuan, Q.; Li, G.; Yan, S. Catalytic Radical- Triggered Annulation/Iododifluoromethylation of Enynones for the Stereospecific Synthesis of 1-Indenones. J. Org. Chem. 2023, 88, 8532–8541; (d) Zhang, S.; Chen, D.; Wang, J.-Y.; Yan, S.; Li, G. Four-layer folding framework: design, GAP synthesis, and aggregation-induced emission. Front. Chem. 2023, 11, 1259609.
- 17(a) Rapisarda, L.; Fermi, A.; Ceroni, P.; Giovanelli, R.; Bertuzzi, G.; Bandini, M. Electrochemical C (sp3)–H functionalization of ethers via hydrogen-atom transfer by means of cathodic reduction. Chem. Commun. 2023, 59, 2664–2667; (b) Senapati, S.; Parida, S. K.; Karandikar, S. S.; Murarka, S. Organophotoredox-Catalyzed Arylation and Aryl Sulfonylation of Morita–Baylis–Hillman Acetates with Diaryliodonium Reagents. Org. Lett. 2023, 25, 7900–7905; (c) Singh, P.; Singh, S.; Rai, V. K.; Yadav, L. D. S. N-Heterocyclic Carbene Catalyzed Cross Coupling of Aromatic Aldehydes with Baylis-Hillman Bromides: An Easy Access to α-Arylidene-γ-keto Esters. Synlett 2010, 17, 2649–2653; (d) Dai, X.; Cheng, D.; Guan, B.; Mao, W.; Xu, X.; Li, X. The coupling of tertiary amines with acrylate derivatives via visible-light photoredox catalysis. J. Org. Chem. 2014, 79, 7212–7219; (e) Ye, H.; Ye, Q.; Cheng, D.; Li, X.; Xu, X. Regioselective oxidative ring-opening of cyclopropenyl carboxylates by visible light photoredox catalysis. Tetrahedron Lett. 2018, 59, 2046–2049.
- 18 Wang, J.-Y.; Li, C.-L.; Xu, T.; Li, M.-F.; Hao, W.-J.; Tu, S.-J.; Wang, J.; Li, G.; Yu, Z.-X.; Jiang, B. Catalytic Enantioselective Construction of 6-4 Ring-Junction All-Carbon Stereocenters and Mechanistic Insights. Chin. J. Chem. 2022, 40, 1767–1776.
- 19(a) Xia, L.; Jin, M.; Jiao, Y.; Yu, S. Synthesis of C-Alkynyl Glycosides by Photoredox-Catalyzed Reductive Coupling of Alkynyl Bromides with Glycosyl Bromides. Org. Lett. 2022, 24, 364–368; (b) Zhang, D.-R.; Hu, L.-P.; Liu, F.-L.; Huang, X.-H.; Li, X.; Liu, B.; Teng, M.-Y.; Huang, G.-L. Catalyst-controlled and visible light-induced acylmethylation and bromoacylmethylation of Morita–Baylis–Hillman acetates with α-bromo ketones: access to highly functionalized 1, 5-dicarbonyl compounds. Org. Chem. Front. 2023, 10, 4927–4934; (c) Zhang, D.-R.; Hu, L.-P.; Liu, F.-L.; Huang, X.-H.; Li, X.; Liu, B.; Huang, G.-L. Metal-free, visible-light-promoted decarboxylative alkylation of Baylis–Hillman acetates with N-(acyloxy) phthalimides. Green Chem. 2022, 24, 6840–6844.
- 20 Constantin, T.; Zanini, M.; Regni, A.; Sheikh, N. S.; Juliá, F.; Leonori, D. Aminoalkyl Radicals as Halogen-Atom Transfer Agents for Activation of Alkyl and Aryl Halides. Science 2020, 367, 1021–1026.




