Transition-Metal-Free Allylic Defluorination Cross-Electrophile Coupling Employing Rongalite
Xiang-Long Chen
National Key Laboratory of Green Pesticide, International Joint Research Center for Intelligent Biosensor Technology and Health, College of Chemistry, Central China Normal University, Wuhan, Hubei, 430079 China
Search for more papers by this authorChun-Yan Wu
National Key Laboratory of Green Pesticide, International Joint Research Center for Intelligent Biosensor Technology and Health, College of Chemistry, Central China Normal University, Wuhan, Hubei, 430079 China
Search for more papers by this authorDong-Sheng Yang
National Key Laboratory of Green Pesticide, International Joint Research Center for Intelligent Biosensor Technology and Health, College of Chemistry, Central China Normal University, Wuhan, Hubei, 430079 China
Search for more papers by this authorBo-Cheng Tang
State Key Laboratory of Chemical Biology and Drug Discovery, Department of Applied Biology and Chemical Technology, The Hong Kong Polytechnic University, Kowloon, Hong Kong, SAR, China
Search for more papers by this authorHuai-Yu Wang
National Key Laboratory of Green Pesticide, International Joint Research Center for Intelligent Biosensor Technology and Health, College of Chemistry, Central China Normal University, Wuhan, Hubei, 430079 China
Search for more papers by this authorZhi-Cheng Yu
National Key Laboratory of Green Pesticide, International Joint Research Center for Intelligent Biosensor Technology and Health, College of Chemistry, Central China Normal University, Wuhan, Hubei, 430079 China
Search for more papers by this authorCorresponding Author
Anling Li
Department of Clinical Laboratory, Center for Gene Diagnosis, and Program of Clinical Laboratory Medicine, Zhongnan Hospital of Wuhan University, Wuhan, Hubei, 430062 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Yan-Dong Wu
National Key Laboratory of Green Pesticide, International Joint Research Center for Intelligent Biosensor Technology and Health, College of Chemistry, Central China Normal University, Wuhan, Hubei, 430079 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
An-Xin Wu
National Key Laboratory of Green Pesticide, International Joint Research Center for Intelligent Biosensor Technology and Health, College of Chemistry, Central China Normal University, Wuhan, Hubei, 430079 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorXiang-Long Chen
National Key Laboratory of Green Pesticide, International Joint Research Center for Intelligent Biosensor Technology and Health, College of Chemistry, Central China Normal University, Wuhan, Hubei, 430079 China
Search for more papers by this authorChun-Yan Wu
National Key Laboratory of Green Pesticide, International Joint Research Center for Intelligent Biosensor Technology and Health, College of Chemistry, Central China Normal University, Wuhan, Hubei, 430079 China
Search for more papers by this authorDong-Sheng Yang
National Key Laboratory of Green Pesticide, International Joint Research Center for Intelligent Biosensor Technology and Health, College of Chemistry, Central China Normal University, Wuhan, Hubei, 430079 China
Search for more papers by this authorBo-Cheng Tang
State Key Laboratory of Chemical Biology and Drug Discovery, Department of Applied Biology and Chemical Technology, The Hong Kong Polytechnic University, Kowloon, Hong Kong, SAR, China
Search for more papers by this authorHuai-Yu Wang
National Key Laboratory of Green Pesticide, International Joint Research Center for Intelligent Biosensor Technology and Health, College of Chemistry, Central China Normal University, Wuhan, Hubei, 430079 China
Search for more papers by this authorZhi-Cheng Yu
National Key Laboratory of Green Pesticide, International Joint Research Center for Intelligent Biosensor Technology and Health, College of Chemistry, Central China Normal University, Wuhan, Hubei, 430079 China
Search for more papers by this authorCorresponding Author
Anling Li
Department of Clinical Laboratory, Center for Gene Diagnosis, and Program of Clinical Laboratory Medicine, Zhongnan Hospital of Wuhan University, Wuhan, Hubei, 430062 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Yan-Dong Wu
National Key Laboratory of Green Pesticide, International Joint Research Center for Intelligent Biosensor Technology and Health, College of Chemistry, Central China Normal University, Wuhan, Hubei, 430079 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
An-Xin Wu
National Key Laboratory of Green Pesticide, International Joint Research Center for Intelligent Biosensor Technology and Health, College of Chemistry, Central China Normal University, Wuhan, Hubei, 430079 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorComprehensive Summary
The conversion of CF3-alkenes to gem-difluoroalkenes using reductive cross-coupling strategy has received much attention in recent years, however, the use of green and readily available reducing salt to mediate these reactions remains to be explored. In this work, a concise construction of gem-difluoroalkenes, which requires neither a catalyst nor a metal reducing agent, was established. Rongalite, a safe and inexpensive industrial product, was employed as both a radical initiator and reductant. This procedure was compatible with both linear and cyclic diaryliodonium salts, enabling a wide variety of substrates (>70 examples). The utility of this approach was demonstrated through gram-scale synthesis and efficient late-stage functionalizations of anti-inflammatory drugs.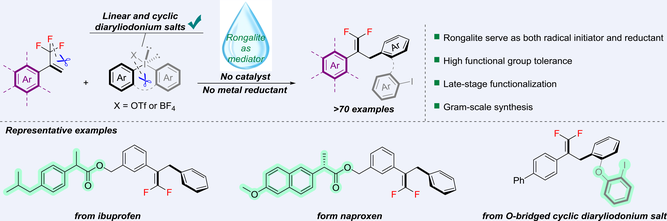
Supporting Information
| Filename | Description |
|---|---|
| cjoc202300751-sup-0001-supinfo.pdfPDF document, 10.8 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Müller, K.; Faeh, C.; Diederich, F. Fluorine in Pharmaceuticals: Looking Beyond Intuition. Science 2007, 317, 1881–1886; (b) Purser, S.; Moore, P. R.; Swallow, S.; Gouverneur, V. Fluorine in Medicinal Chemistry. Chem. Soc. Rev. 2008, 37, 320–330; (c) Hagmann, W. K. The Many Roles for Fluorine in Medicinal Chemistry. J. Med. Chem. 2008, 51, 4359–4369; (d) Zhou, Y.; Wang, J.; Gu, Z.; Wang, S.; Zhu, W.; Aceña, J. L.; Soloshonok, V. A.; Izawa, K.; Liu, H. Next Generation of Fluorine-Containing Pharmaceuticals, Compounds Currently in Phase II-III Clinical Trials of Major Pharmaceutical Companies: New Structural Trends and Therapeutic Areas. Chem. Rev. 2016, 116, 422–518.
- 2(a) Madden, B. A.; Prestwich, G. D. Potency and Inactivation Rates of Analogues of an Irreversible Inhibitor of Vertebrate Oxidosqualene Cyclase. Bioorg. Med. Chem. Lett. 1997, 7, 309–314; (b) Weintraub, P. M.; Holland, A. K.; Gates, C. A.; Moore, W. R.; Resvick, R. J.; Bey, P.; Peet, N. P. Synthesis of 21,21-Difluoro-3β-hydroxy-20-methylpregna- 5,20-diene and 5,16,20-Triene as Potential Inhibitors of Steroid C17(20) Lyase. Bioorg. Med. Chem. 2003, 11, 427–431; (c) Altenburger, J. M.; Lassalle, G. Y.; Matrougui, M.; Galtier, D.; Jetha, J. C.; Bocskei, Z.; Berry, C. N.; Lunven, C.; Lorrain, J.; Herault, J. P.; Schaeffer, P.; O'Connor, S. E.; Herbert, J. M. SSR182289A, a Selective and Potent Orally Active Thrombin Inhibitor. Bioorg. Med. Chem. 2004, 12, 1713–1730; (d) Messaoudi, S.; Tréguier, B.; Hamze, A.; Provot, O.; Peyrat, J. F.; De Losada, J. R.; Liu, J. M.; Bignon, J.; Wdzieczak-Bakala, J.; Thoret, S.; Dubois, J.; Brion, J. D.; Alami, M. Isocombretastatins A Versus Combretastatins A: The Forgotten isoCA-4 Isomer as a Highly Promising Cytotoxic and Antitubulin Agent. J. Med. Chem. 2009, 52, 4538–4542.
- 3(a) McDonald, I. A.; Lacoste, J. M.; Bey, P.; Palfreyman, M. G.; Zreika, M. Enzyme-activated Irreversible Inhibitors of Monoamine Oxidase: Phenylallylamine Structure-activity Relationships. J. Med. Chem. 1985, 28, 186–193; (b) Bobek, M.; Kavai, I.; De Clercq, E. Synthesis and Biological Activity of 5-(2,2-difluorovinyl)-2'-deoxyuridine. J. Med. Chem. 1987, 30, 1494–1497; (c) Pan, Y.; Qiu, J.; Silverman, R. B. Design, Synthesis, and Biological Activity of a Difluoro-Substituted, Conformationally Rigid Vigabatrin Analogue as a Potent γ-Aminobutyric Acid Aminotransferase Inhibitor. J. Med. Chem. 2003, 46, 5292–5293; (d) Magueur, G.; Crousse, B.; Ourévitch, M.; Bonnet-Delpon, D.; Bégué, J. P. Fluoro-artemisinins: When a Gem-difluoroethylene Replaces a Carbonyl Group. J. Fluorine Chem. 2006, 127, 637–642; (e) Zhang, X.; Cao, S. Recent Advances in the Synthesis and C-F Functionalization of gem-difluoroalkenes. Tetrahedron Lett. 2017, 58, 375–392.
- 4(a) Bégué, J. P.; Bonnet-Delpon, D.; Rock, M. H. A Concise Synthesis of Functionalised gem-difluoroalkenes, via the Addition of Organolithium Reagents to α-trifluoromethylstyrene. Tetrahedron Lett. 1995, 36, 5003–5006; (b) Ichikawa, J.; Fukui, H.; Ishibashi, Y. 1-Trifluoromethylvinylsilane as a CF2=C--CH2+ Synthon: Synthesis of Functionalized 1,1-Difluoro-1-alkenes via Isolable 2,2-Difluorovinylsilanes. J. Org. Chem. 2003, 68, 7800–7805; (c) Fuchibe, K.; Takahashi, M.; Ichikawa, J. Substitution of Two Fluorine Atoms in a Trifluoromethyl Group: Regioselective Synthesis of 3-Fluoropyrazoles. Angew. Chem. Int. Ed. 2012, 51, 12059–12062; (d) Fuchibe, K.; Hatta, H.; Oh, K.; Oki, R.; Ichikawa, J. Lewis Acid Promoted Single C-F Bond Activation of the CF3 Group: SN1’-Type 3,3-Difluoroallylation of Arenes with 2-Trifluoromethyl-1-alkenes. Angew. Chem. Int. Ed. 2017, 56, 5890–5893; (e) Dai, W.; Lin, Y.; Wan, Y.; Cao, S. Cu-Catalyzed Tertiary Alkylation of α-(trifluoromethyl)styrenes with Tertiary Alkylmagnesium Reagents. Org. Chem. Front. 2018, 5, 55–58; (f) Cai, Y.; Zeng, H.; Zhu, C.; Liu, C.; Liu, G.; Jiang, H. Double Allylic Defluorinative Alkylation of 1,1-bisnucleophiles with (trifluoromethyl)alkenes: Construction of All-carbon Quaternary Centers. Org. Chem. Front. 2020, 7, 1260–1265; (g) Huang, H.; Chen, J.; Jiang, Y.; Xiao, T. One Pot Synthesis of Isocyano- containing, Densely Functionalised gem-difluoroalkenes from α-trifluoromethyl Alkenes, Alkyl Halides and TosMIC. Org. Chem. Front. 2021, 8, 5955–5961; (h) Kim, H.; Jung, Y.; Cho, S. H. Defluorinative C-C Bond-Forming Reaction of Trifluoromethyl Alkenes with gem-(Diborylalkyl)lithiums. Org. Lett. 2022, 24, 2705–2710; (i) Chu, X. Q.; Sun, L. W.; Chen, Y. L.; Chen, J. W.; Ying, X.; Ma, M.; Shen, Z. L. HP(O)Ph2/H2O-promoted Hydrodefluorination of Trifluoromethyl Alkenes. Green Chem. 2022, 24, 2777–2782; (j) Rahman, A. J. M.; Xu, Y.; Oestreich, M. Fluoride-Catalyzed Arylation of α-(Trifluoromethyl)styrene Derivatives with Silicon-Masked, Functionalized Aryl Pronucleophiles. Org. Lett. 2023, 25, 5636–5640; (k) Chen, X. L.; Yang, D. S.; Tang, B. C.; Wu, C. Y.; Wang, H. Y.; Ma, J. T.; Zhuang, S. Y.; Yu, Z. C.; Wu, Y. D.; Wu, A. X. Direct Hydrodefluorination of CF3-Alkenes via a Mild SN2’ Process Using Rongalite as a Masked Proton Reagent. Org. Lett. 2023, 25, 2294–2299; (l) Xing, W.; Wang, J.; Fu, M.; Fu, Y. Efficient Decarboxylative/Defluorinative Alkylation for the Synthesis of gem-Difluoroalkenes Through an SN2’-Type Route. Chin. J. Chem. 2021, 40, 323–328.
- 5(a) Yao, C.; Wang, S.; Norton, J.; Hammond, M. Catalyzing the Hydrodefluorination of CF3-Substituted Alkenes by PhSiH3 H• Transfer from a Nickel Hydride. J. Am. Chem. Soc. 2020, 142, 4793–4799;
(b) Ding, D.; Lan, Y.; Lin, Z.; Wang, C. Synthesis of gem-Difluoroalkenes by Merging Ni-Catalyzed C-F and C-C Bond Activation in Cross-Electrophile Coupling. Org. Lett. 2019, 21, 2723–2730;
(c) Lin, Z.; Lan, Y.; Wang, C. Synthesis of gem-Difluoroalkenes via Nickel-Catalyzed Reductive C-F and C-O Bond Cleavage. ACS Catal. 2018, 9, 775–780;
(d) Chen, F.; Xu, X.; He, Y.; Huang, G.; Zhu, S. NiH-Catalyzed Migratory Defluorinative Olefin Cross-Coupling: Trifluoromethyl-Substituted Alkenes as Acceptor Olefins to Form gem-Difluoroalkenes. Angew. Chem. Int. Ed. 2020, 59, 5398–5402;
(e) Zhu, C.; Liu, Z. Y.; Tang, L.; Zhang, H.; Zhang, Y. F.; Walsh, P. J.; Feng, C. Migratory Functionalization of Unactivated Alkyl Bromides for Construction of All-carbon Quaternary Centers via Transposed tert-C-radicals. Nat. Commun. 2020, 11, 4860;
(f) Lin, Z.; Lan, Y.; Wang, C. Titanocene-Catalyzed Reductive Domino Epoxide Ring Opening/Defluorinative Cross-Coupling Reaction. Org. Lett. 2020, 22, 3509–3514;
(g) Du, H. W.; Chen, Y.; Sun, J.; Gao, Q. S.; Wang, H.; Zhou, M. D. Synthesis of gem-Difluoroalkenes via Zn-Mediated Decarboxylative/Defluorinative Cross- Coupling. Org. Lett. 2020, 22, 9342–9345;
(h) Dong, H.; Lin, Z.; Wang, C. Nickel-Catalyzed Allylic Defluorinative Cross-Electrophile Coupling with Cycloalkyl Silyl Peroxides as the Alkyl Source. J. Org. Chem. 2021, 87, 892–903;
(i) Dong, H.; Lin, Z.; Wang, C. Cobalt-Catalyzed Allylic Defluorinative Cross-Electrophile Coupling Between 1,1-Difluoroalkyl Halides and α-Trifluoromethyl Styrenes. Adv. Synth. Catal. 2023, 365, 1165–1169;
(j) Yuan, B.; Zhang, C.; Dong, H.; Wang, C. Iron-Catalyzed Reductive Ring Opening/gem-Difluoroallylation of Cyclopropyl Ketones. Org. Lett. 2023, 25, 1883–1888;
(k) Luo, H.; Zhao, Y.; Wang, D.; Wang, M.; Shi, Z. Stereoselective Fluoroarylation of 1,1-difluoroallenes Enabled by Palladium Catalysis. Green Synth. Catal. 2020, 1, 134–142;
10.1016/j.gresc.2020.08.002 Google Scholar(l) Mao, Y.; Liu, Y.; Wang, X.; Ni, S.; Pan, Y.; Wang, Y. Acylfluorination of Enynes via Phosphine and Silver Catalysis. Chin. Chem. Lett. 10.1016/j.cclet.2023.109443; (m) Wu, F.; Li, X.; Chang, J.; Bai, D. Palladium-catalyzed Multi Components Oxy-aminofluorination and Aminofluorination of Gem-difluoroalkenes. Chin. Chem. Lett. 2024, 35, 109155.
- 6(a) Lang, S. B.; Wiles, R. J.; Kelly, C. B.; Molander, G. A. Photoredox Generation of Carbon-Centered Radicals Enables the Construction of 1,1-Difluoroalkene Carbonyl Mimics. Angew. Chem. Int. Ed. 2017, 56, 15073–15077;
(b) Phelan, J. P.; Lang, S. B.; Sim, J.; Berritt, S.; Peat, A. J.; Billings, K.; Fan, L.; Molander, G. A. Open-Air Alkylation Reactions in Photoredox-Catalyzed DNA-Encoded Library Synthesis. J. Am. Chem. Soc. 2019, 141, 3723–3732;
(c) Xu, W.; Jiang, H.; Leng, J.; Ong, H.; Wu, J. Visible-Light-Induced Selective Defluoroborylation of Polyfluoroarenes, gem-Difluoroalkenes, and Trifluoromethylalkenes. Angew. Chem. Int. Ed. 2020, 132, 4038–4045;
10.1002/ange.201911819 Google Scholar(d) He, Y.; Anand, D.; Sun, Z.; Zhou, L. Visible-Light-Promoted Redox Neutral γ,γ-Difluoroallylation of Cycloketone Oxime Ethers with Trifluoromethyl Alkenes via C-C and C-F Bond Cleavage. Org. Lett. 2019, 21, 3769–3773; (e) Yue, W. J.; Day, C. S.; Martin, R. Site-Selective Defluorinative Sp3 C-H Alkylation of Secondary Amides. J. Am. Chem. Soc. 2021, 143, 6395–6400; (f) Hu, Q. P.; Cheng, J.; Wang, Y.; Shi, J.; Wang, B. Q.; Hu, P.; Zhao, K. Q.; Pan, F. Remote Regioselective Radical C-H Functionalization of Unactivated C-H Bonds in Amides: The Synthesis of gem-Difluoroalkenes. Org. Lett. 2021, 23, 4457–4462; (g) Guo, Y. Q.; Wu, Y.; Wang, R.; Song, H.; Liu, Y.; Wang, Q. Photoredox/Hydrogen Atom Transfer Cocatalyzed C-H Difluoroallylation of Amides, Ethers, and Alkyl Aldehydes. Org. Lett. 2021, 23, 2353–2358; (h) Gao, Q. S.; Niu, Z.; Chen, Y.; Sun, J.; Han, W. Y.; Wang, J. Y.; Yu, M.; Zhou, M. D. Photoredox Generation of N-Centered Hydrazonyl Radicals Enables the Construction of Dihydropyrazole-Fused gem-Difluoroalkenes. Org. Lett. 2021, 23, 6153–6157; (i) Wang, J. X.; Ge, W.; Fu, M. C.; Fu, Y. Photoredox-Catalyzed Allylic Defluorinative Alkoxycarbonylation of Trifluoromethyl Alkenes Through Intermolecular Alkoxycarbonyl Radical Addition. Org. Lett. 2022, 24, 1471–1475; (j) Xia, G. D.; He, Y. Y.; Zhang, J.; Liu, Z. K.; Gao, Y.; Hu, X. Q. Deoxygenative gem-difluorovinylation of Aliphatic Alcohols. Chem. Commun. 2022, 58, 6733–6736; (k) Li, W.; Chen, X.; Zhou, L. Photocatalytic Defluorinative Three-Component Reaction of α-Trifluoromethyl Alkenes, Alkenes, and Sodium Sulfinates: Synthesis of Monofluorocyclopentenes. Org. Lett. 2022, 24, 5946–5950; (l) Xu, Y.; Wang, S.; Liu, Z.; Guo, M.; Lei, A. Photo/Ni Dual-catalyzed Radical Defluorinative Sulfonylation to Synthesize gem-difluoro Allylsulfones. Chem. Commun. 2023, 59, 3707–3710; (m) Qin, T.; Xu, C.; Zhang, G.; Zhang, Q. Visible-light-promoted Defluorinated Alkylation of Trifluoromethyl Alkenes Initiated by Radical [1,2]-Brook Rearrangement: Facile Synthesis of gem-difluoro Homoallylic Alcohol Derivatives. Org. Chem. Front. 2023, 10, 1981–1987; (n) Tian, J.; Zhou, L. Photoredox Radical/polar Crossover Enables C-H gem-difunctionalization of 1,3-benzodioxoles for the Synthesis of Monofluorocyclohexenes. Chem. Sci. 2023, 14, 6045–6051; (o) Zhang, Y.; Zhang, Y.; Guo, Y.; Liu, S.; Shen, X. Reductive Quenching-initiated Catalyst-controlled Divergent Alkylation of α-CF3-olefins. Chem Catal. 2022, 2, 1380–1393;10.1016/j.checat.2022.03.021 Google Scholar(p) Zhang, Y.; Niu, Y.; Guo, Y.; Wang, J.; Zhang, Y.; Liu, S.; Shen, X. Photocatalyzed Cascade Reactions of Cyclopropanols and α-Trifluoromethyl-Substituted Olefins for the Synthesis of Fused gem-Difluorooxetanes. Angew. Chem. Int. Ed. 2022, 61, e202212201; (q) Li, Z.; Zhang, Y.; Zhang, Y.; He, X.; Shen, X. Diastereoselective Synthesis of Monofluorocyclohexenes Through Photocatalyzed Cascade Cyclization of gem-Difluoroalkenes and α,β-Unsaturated Carbonyl Compounds. Angew. Chem. Int. Ed. 2023, 62, e202303218; (r) Zhang, Y.; Wang, J.; Guo, Y.; Liu, S.; Shen, X. Carbonyl Olefin Metathesis and Dehydrogenative Cyclization of Aromatic Ketones and gem-Difluoroalkenes. Angew. Chem. Int. Ed. 2024, 63, e202315269; (s) Chen, S.; Zhang, Y.; Liu, S.; Shen, X. Photocatalyzed [2+1] Cyclization of Alkenes and Silylated Trifluorodiazoethanes: Facile Entry into (difluoromethylene)cyclopropanes. Sci. China Chem. 2023, 66, 3141–3147.
- 7(a) Gao, X. T.; Zhang, Z.; Wang, X.; Tian, J. S.; Xie, S. L.; Zhou, F.; Zhou, J. Direct Electrochemical Defluorinative Carboxylation of α-CF3 Alkenes with Carbon Dioxide. Chem. Sci. 2020, 11, 10414–10420; (b) Liu, Y.; Tao, X.; Mao, Y.; Yuan, X.; Qiu, J.; Kong, L.; Ni, S.; Guo, K.; Wang, Y.; Pan, Y. Electrochemical C-N Bond Activation for Deaminative Reductive Coupling of Katritzky Salts. Nat. Commun. 2021, 12, 6745.
- 8(a) Lan, Y.; Yang, F.; Wang, C. Synthesis of gem-Difluoroalkenes via Nickel-Catalyzed Allylic Defluorinative Reductive Cross-Coupling. ACS Catal. 2018, 8, 9245–9251; (b) Lu, X.; Wang, X. X.; Gong, T. J.; Pi, J. J.; He, S. J.; Fu, Y. Nickel-catalyzed Allylic Defluorinative Alkylation of Trifluoromethyl Alkenes with Reductive Decarboxylation of Redox-active Esters. Chem. Sci. 2019, 10, 809–814; (c) Lin, Z.; Lan, Y.; Wang, C. Reductive Allylic Defluorinative Cross-Coupling Enabled by Ni/Ti Cooperative Catalysis. Org. Lett. 2019, 21, 8316–8322; (d) Lu, X. Y.; Jiang, R. C.; Li, J. M.; Liu, C. C.; Wang, Q. Q.; Zhou, H. P. Synthesis of gem-difluoroalkenes via Nickel-catalyzed Allylic Defluorinative Reductive Cross-coupling of Trifluoromethyl Alkenes with Epoxides. Org. Biomol. Chem. 2020, 18, 3674–3678; (e) Jin, Y.; Wu, J.; Lin, Z.; Lan, Y.; Wang, C. Merger of C-F and C-N Bond Cleavage in Cross- Electrophile Coupling for the Synthesis of gem-Difluoroalkenes. Org. Lett. 2020, 22, 5347–5352; (f) Zhang, C.; Lin, Z.; Zhu, Y.; Wang, C. Chromium-Catalyzed Allylic Defluorinative Ketyl Olefin Coupling. J. Am. Chem. Soc. 2021, 143, 11602–11610; (g) Ma, T.; Li, X.; Ping, Y.; Kong, W. Synthesis of gem-Difluoroalkenes via Ni-Catalyzed Three- Component Defluorinative Reductive Cross-Coupling of Organohalides, Alkenes and Trifluoromethyl Alkenes. Chin. J. Chem. 2022, 40, 2212–2218; (h) Qiu, J.; Wang, C.; Zhou, L.; Lou, Y.; Yang, K.; Song, Q. Ni-Catalyzed Radical-Promoted Defluoroalkylborylation of Trifluoromethyl Alkenes to Access gem-Difluorohomoallylic Boronates. Org. Lett. 2022, 24, 2446–2451; (i) Zhang, C.; Wang, L.; Shi, H.; Lin, Z.; Wang, C. Iron-Catalyzed Allylic Defluorinative Ketone Olefin Coupling. Org. Lett. 2022, 24, 3211–3216; (j) Tian, H.; Zhang, R.; Shi, L.; Zhao, C.; Wang, X. Divergent Synthesis of Organofluorinated Molecules from Titanium Mediated Deoxygenation of Free Alcohols. Chin. J. Chem. 2023, 41, 1783–1790.
- 9(a) Wiles, R. J.; Phelan, J. P.; Molander, G. A. Metal-free Defluorinative Arylation of Trifluoromethyl Alkenes via Photoredox Catalysis. Chem. Commun. 2019, 55, 7599–7602; (b) Li, C. Y.; Ma, Y.; Lei, Z. W.; Hu, X. G. Glycosyl-Radical-Based Synthesis of C-Alkyl Glycosides via Photomediated Defluorinative gem-Difluoroallylation. Org. Lett. 2021, 23, 8899–8904; (c) Guo, Y.; Cao, Y.; Song, H.; Liu, Y.; Wang, Q. Photoredox Relay-catalyzed gem-difluoroallylation of Alkyl Iodides. Chem. Commun. 2021, 57, 9768–9771; (d) Cai, Z.; Gu, R.; Si, W.; Xiang, Y.; Sun, J.; Jiao, Y.; Zhang, X. Photoinduced Allylic Defluorinative Alkylation of Trifluoromethyl Alkenes with Katritzky Salts Under Catalyst- and Metal-free Conditions. Green Chem. 2022, 24, 6830–6835; (e) Yan, S.; Yu, W.; Zhang, J.; Fan, H.; Lu, Z.; Zhang, Z.; Wang, T. Access to gem-Difluoroalkenes via Organic Photoredox-Catalyzed gem-Difluoroallylation of Alkyl Iodides. J. Org. Chem. 2021, 87, 1574–1584; (f) Wang, B.; Wang, C. T.; Li, X. S.; Liu, X. Y.; Liang, Y. M. Visible-Light-Induced C-F and C-N Bond Cleavage for the Synthesis of gem-Difluoroalkenes. Org. Lett. 2022, 24, 6566–6570; (g) Zhao, Y.; Empel, C.; Liang, W.; Koenigs, R. M.; Patureau, F. W. gem-Difluoroallylation of Aryl Sulfonium Salts. Org. Lett. 2022, 24, 8753–8758; (h) Chen, B.; Yu, K.; Wu, X. F. Visible-light-induced Defluorinative Carbonylative Coupling of Alkyl Iodides with α-trifluoromethyl Substituted Styrenes. Org. Biomol. Chem. 2022, 20, 5264–5269.
- 10(a) Claraz, A.; Allain, C.; Masson, G. Electroreductive Cross-Coupling of Trifluoromethyl Alkenes and Redox Active Esters for the Synthesis of gem-Difluoroalkenes. Chem. Eur. J. 2021, 28, e202103337; (b) Zhang, H.; Liang, M.; Zhang, X.; He, M. K.; Yang, C.; Guo, L.; Xia, W. Electrochemical Synthesis of Functionalized gem-difluoroalkenes with Diverse Alkyl Sources via a Defluorinative Alkylation Process. Org. Chem. Front. 2022, 9, 95–101; (c) Yan, X.; Wang, S.; Liu, Z.; Luo, Y.; Wang, P.; Shi, W.; Qi, X.; Huang, Z.; Lei, A. Precise Electro-reduction of Alkyl Halides for Radical Defluorinative Alkylation. Sci. China Chem. 2022, 65, 762–770; (d) Chen, W.; Ni, S.; Wang, Y.; Pan, Y. Electrochemical-Promoted Nickel-Catalyzed Reductive Allylation of Aryl Halides. Org. Lett. 2022, 24, 3647–3651.
- 11 Meng, Y.; Wang, M.; Jiang, X. Transition-Metal-Free Reductive Cross-Coupling Employing Metabisulfite as a Connector: General Construction of Alkyl-Alkyl Sulfones. CCS Chem. 2021, 3, 17–24.
- 12(a) Wang, M.; Tang, B. C.; Wang, J. G.; Xiang, J. C.; Guan, A. Y.; Huang, P. P.; Guo, W. Y.; Wu, Y. D.; Wu, A. X. The Triple Role of Rongalite in Aminosulfonylation of Aryldiazonium Tetrafluoroborates: Synthesis of N-aminosulfonamides via a Radical Coupling Reaction. Chem. Commun. 2018, 54, 7641–7644; (b) Wang, M.; Tang, B. C.; Xiang, J. C.; Chen, X. L.; Ma, J. T.; Wu, Y. D.; Wu, A. X. Aryldiazonium Salts Serve as a Dual Synthon: Construction of Fully Substituted Pyrazoles via Rongalite-Mediated Three-Component Radical Annulation Reaction. Org. Lett. 2019, 21, 8934–8937; (c) Chen, X. L.; Tang, B. C.; He, C.; Ma, J. T.; Zhuang, S. Y.; Wu, Y. D.; Wu, A. X. Rongalite as a Sulfone Source: a Novel Copper-catalyzed Sulfur Dioxide Anion Incorporation Process. Chem. Commun. 2020, 56, 13653–13656; (d) Alvarez, E. M.; Plutschack, M. B.; Berger, F.; Ritter, T. Site-Selective C-H Functionalization-Sulfination Sequence to Access Aryl Sulfonamides. Org. Lett. 2020, 22, 4593–4596; (e) Chen, X. L.; Wu, C. Y.; Ma, J. T.; Zhuang, S. Y.; Yu, Z. C.; Wu, Y. D.; Wu, A. X. Rongalite as C1 Synthon and Sulfone Source: A Practical Sulfonylmethylation Based on the Separate-Embedding Strategy. Org. Lett. 2021, 24, 223–227; (f) Chen, X. L.; Wang, H. Y.; Wu, C. Y.; Tang, B. C.; Hu, Y. L.; Ma, J. T.; Zhuang, S. Y.; Yu, Z. C.; Wu, Y. D.; Wu, A. X. Synthesis of Tetrahydro-2H-thiopyran 1,1-Dioxides via [1+1+1+1+1+1] Annulation: An Unconventional Usage of a Tethered C-S Synthon. Org. Lett. 2022, 24, 7659–7664; (g) Wang, H. Y.; Chen, X. L.; Wu, C. Y.; Yang, D. S.; Chen, T.; Wu, A. X. Reductive N-Formylation of Nitroarenes Mediated by Rongalite. Org. Lett. 2023, 25, 7220–7224; (h) Wang, H. Y.; Chen, X. L.; Wu, Y. D.; Wu, A. X. Rongalite as a Versatile Reagent in Organic Synthesis. Chin. J. Chem. 2023, 41, 3388–3400; (i) Zhang, W.; Luo, M. Iron-catalyzed Synthesis of Arylsulfinates Through Radical Coupling Reaction. Chem. Commun. 2016, 52, 2980–2983; (j) Yu, F.; Mao, R.; Yu, M.; Gu, X.; Wang, Y. Generation of Aryl Radicals from Aryl Halides: Rongalite-Promoted Transition-Metal-Free Arylation. J. Org. Chem. 2019, 84, 9946–9956; (k) Laha, J. K.; Gupta, P. Sulfoxylate Anion Radical-Induced Aryl Radical Generation and Intramolecular Arylation for the Synthesis of Biarylsultams. J. Org. Chem. 2022, 87, 4204–4214; (l) Golla, S.; Kokatla, H. P. Rongalite-Mediated Transition Metal- and Hydride-Free Chemoselective Reduction of α-Keto Esters and α-Keto Amides. J. Org. Chem. 2022, 87, 9915–9925.




