Synthesis of Diverse Oxetane Amino Acids via Visible-Light-Induced Photocatalytic Decarboxylative Giese-Type Reaction
Haoliang Shi
Shanghai Frontiers Science Center of Optogenetic Techniques for Cell Metabolism, Shanghai Key Laboratory of New Drug Design, and School of Pharmacy, East China University of Science and Technology, Shanghai, 200237 China
Search for more papers by this authorYi Wan
Shanghai Frontiers Science Center of Optogenetic Techniques for Cell Metabolism, Shanghai Key Laboratory of New Drug Design, and School of Pharmacy, East China University of Science and Technology, Shanghai, 200237 China
Search for more papers by this authorCorresponding Author
Yongqiang Zhang
Shanghai Frontiers Science Center of Optogenetic Techniques for Cell Metabolism, Shanghai Key Laboratory of New Drug Design, and School of Pharmacy, East China University of Science and Technology, Shanghai, 200237 China
E-mail: [email protected]Search for more papers by this authorHaoliang Shi
Shanghai Frontiers Science Center of Optogenetic Techniques for Cell Metabolism, Shanghai Key Laboratory of New Drug Design, and School of Pharmacy, East China University of Science and Technology, Shanghai, 200237 China
Search for more papers by this authorYi Wan
Shanghai Frontiers Science Center of Optogenetic Techniques for Cell Metabolism, Shanghai Key Laboratory of New Drug Design, and School of Pharmacy, East China University of Science and Technology, Shanghai, 200237 China
Search for more papers by this authorCorresponding Author
Yongqiang Zhang
Shanghai Frontiers Science Center of Optogenetic Techniques for Cell Metabolism, Shanghai Key Laboratory of New Drug Design, and School of Pharmacy, East China University of Science and Technology, Shanghai, 200237 China
E-mail: [email protected]Search for more papers by this authorComprehensive Summary
The divergent synthesis of versatile 3,3′-disubstituted oxetane amino acids by utilizing visible-light-induced photocatalytic decarboxylative Giese-type reaction has been demonstrated. 3-Methyleneoxetane-derived substrates are readily available in a single-step and highly reactive as radical acceptors, allowing the production of versatile oxetane γ- and α-amino acids in high yields. A distinct ring strain release-driven radical addition mechanism was preliminarily revealed. The preparative power was further highlighted by the application in the synthesis of oxetane-containing dipeptides and azetidine amino acids, as well as the transformation of the product into novel oxetane-containing spiro-heterocycle pharmacophore.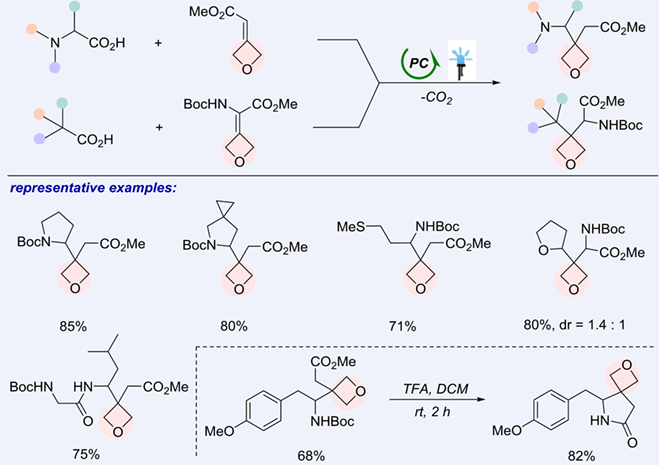
Supporting Information
| Filename | Description |
|---|---|
| cjoc202300750-sup-0001-supinfo.pdfPDF document, 4.4 MB |
Appendix S1: Supporting Inforamtion |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Veber, D. F.; Holly, F. W.; Nutt, R. F.; Bergstrand, S. J.; Saperstein, R.; Hisrschmann, R.; Glitzer, M. S.; Saperstein, R. Highly Active Cyclic and Bicyclic Somatostatin Analogues of Reduced Ring Size. Nature 1979, 280, 512–514.
- 2 Wuitschik, G.; Carreira, E. M.; Wagner, B.; Fischer, H.; Parrilla, I.; Schuler, F.; Evans, M. R.; Müller, K. Oxetanes in Drug Discovery: Structural and Synthetic Insights. J. Med. Chem. 2010, 53, 3227–3246.
- 3 Rojas, J. J.; Bull, J. A. Oxetanes in Drug Discovery Campaigns. J. Med. Chem. 2023, 66, 12697–12709.
- 4 Kolahdouzan, K.; Khalaf, R.; Grandner, J. M.; Chen, Y. S.; Terrett, J. A.; Huestis, M. P. Dual Photoredox/Nickel-Catalyzed Conversion of Aryl Halides to Aryl Aminooxetanes: Computational Evidence for a Substrate-Dependent Switch in Mechanism. ACS Catal. 2020, 10, 405–411.
- 5 Kawahata, Y.; Takatsuto, S.; Ikekawa, N.; Murata, M.; Omura, S. Synthesis of a New Amino Acid-Antibiotic, Oxetin and Its Three Stereoisomers. Chem. Pharm. Bull. 1986, 34, 3102–3110.
- 6 Blauvelt, M. L.; Howell, A. R. Synthesis of epi-Oxetin via a Serine-Derived 2-Methyleneoxetane. J. Org. Chem. 2008, 73, 517–521.
- 7 Kassir, A. F.; Ragab, S. S.; Nguyen, T. A. M.; Pouget, F. C.; Guillot, R.; Scherrmann, M. C.; Boddaert, T.; Aitken, D. J. Synthetic Access to All Four Stereoisomers of Oxetin. J. Org. Chem. 2016, 81, 9983–9991.
- 8 Sharma, G. V. M.; Venkateshwarlu, G.; Katukuri, S.; Ramakrishna, K. V. S.; Sarma, A. V. S. Design and Synthesis of Novel Oxetane β3-Amino Acids and α,β-Peptides. Tetrahedron 2015, 71, 2158–2167.
- 9 Lucas, S. D.; Iding, H.; Alker, A.; Wessel, H. P. Oxetane δ-Amino Acids: Chemoenzymatic Synthesis of 2,4-Anhydro-5-N-(t-butoxycarbonyl)amino-D-lyxonic Acid. Carbohyd. Chem. 2006, 25, 187–196.
- 10 Lucas, S. D.; Rauter, A. P.; Schneider, J.; Wessel, H. P. Synthesis of 3-Fluoro-Oxetane δ-Amino Acids. Carbohyd. Chem. 2009, 28, 431–446.
- 11
Kozikowski, A. P.; Fauq, A. H. Synthesis of Novel Four-Membered Ring Amino Acids as Modulators of the N-Methyl-D-Aspartate (NMDA) Receptor Complex. Synlett 1991, 1991, 783–784.
10.1055/s-1991-20873 Google Scholar
- 12 Burkhard, J. A.; Guérot, C.; Knust, H.; Carreira, E. M. Expanding the Azaspiro[3.3]heptane Family: Synthesis of Novel Highly Functionalized Building Blocks. Org. Lett. 2012, 14, 66–69.
- 13 Gudelis, E.; Krikštolaitytė, S.; Stančiauskaitė, M.; Šachlevičiūtė, U.; Bieliauskas, A.; Milišiūnaitė, V.; Jankauskas, R.; Kleizienė, N.; Sløk, A. F.; Šačkus, A. Synthesis of New Azetidine and Oxetane Amino Acid Derivatives through Aza-Michael Addition of NH-Heterocycles with Methyl 2-(Azetidin- or Oxetan-3-Ylidene)Acetates. Molecules 2023, 28, 1091–1121.
- 14 McCarver, S. J.; Qiao, J. X.; Carpenter, J.; Borzilleri, R. M.; Poss, M. A.; Eastgate, M. D.; Miller, M. M.; MacMillan, D. W. C. Decarboxylative Peptide Macrocyclization through Photoredox Catalysis. Angew. Chem. Int. Ed. 2017, 56, 728–732.
- 15 Bloom, S.; Liu, C.; Kölmel, D. K.; Qiao, J. X.; Zhang, Y.; Poss, M. A.; Ewing, W. R.; MacMillan, D. W. C. Decarboxylative Alkylation for Site-Selective Bioconjugation of Native Proteins via Oxidation Potentials. Nat. Chem. 2017, 10, 205–211.
- 16 Noble, A.; Mega, R. S.; Pflästerer, D.; Myers, E. L.; Aggarwal, V. K. Visible-Light-Mediated Decarboxylative Radical Additions to Vinyl Boronic Esters: Rapid Access to γ-Amino Boronic Esters. Angew. Chem. Int. Ed. 2018, 57, 2155–2159.
- 17 Aycock, R. A.; Pratt, C. J.; Jui, N. T. Aminoalkyl Radicals as Powerful Intermediates for the Synthesis of Unnatural Amino Acids and Peptides. ACS Catal. 2018, 8, 9115–9119.
- 18 Liu, X.; Yin, Y.; Jiang, Z. Photoredox-Catalysed Formal [3+2] Cycloaddition of N-aryl α-Amino Acids with Isoquinoline N-oxides. Chem. Commun. 2019, 55, 11527–11530.
- 19 Gueret, R.; Pelinski, L.; Bousquet, T.; Sauthier, M.; Ferey, V.; Bigot, A. Visible-Light-Driven CarboxyLic Amine Protocol (CLAP) for the Synthesis of 2-Substituted Piperazines under Batch and Flow Conditions. Org. Lett. 2020, 22, 5157–5162.
- 20 Liao, L. L.; Cao, G. M.; Jiang, Y. X.; Jin, X. H.; Hu, X. L.; Chruma, J. J.; Sun, G. Q.; Gui, Y. Y.; Yu, D. G. α-Amino Acids and Peptides as Bifunctional Reagents: Carbocarboxylation of Activated Alkenes via Recycling CO2. J. Am. Chem. Soc. 2021, 143, 2812–2821.
- 21 Zhou, C.; Li, M.; Sun, J. W.; Cheng, J.; Sun, S. Photoredox-Catalyzed α-Aminomethyl Carboxylation of Styrenes with Sodium Glycinates: Synthesis of γ-Amino Acids and γ-Lactams. J. Org. Lett. 2021, 23, 2895–2899.
- 22 Hu, W. G.; Zhan, Q. Q.; Zhou, H. W.; Cao, S. S.; Jiang, Z. Y. Radical-Based Functionalization-Oriented Construction: Rapid Assembly of Azaarene-substituted Highly Functionalized Pyrroles. Chem. Sci. 2021, 12, 6543–6550.
- 23 Deng, Y. P.; He, J. J.; Cao, S.; Qian, X. H. Advances in Cycloaddition and Hydroaddition Reaction of α-(trifluoromethyl)Styrenes without Defluorination: An Alternative Approach to CF3-Containing Compounds. Chin. Chem. Lett. 2022, 33, 2363–2371.
- 24 Sun, Z. Z.; Huang, H. W.; Wang, Q. L.; Deng, G. J. Bromo Radical-Mediated Photoredox Aldehyde Decarbonylation towards Transition-Metal-Free Hydroalkylation of Acrylamides at Room Temperature. Adv. Synth. Catal. 2022, 364, 453–458.
- 25 Ji, P.; Chen, J.; Meng, X.; Gao, F.; Dong, Y.; Xu, H.; Wang, W. Design of Photoredox-Catalyzed Giese-Type Reaction for the Synthesis of Chiral Quaternary α-Aryl Amino Acid Derivatives via Clayden Rearrangement. J. Org. Chem. 2022, 87, 14706–14714.
- 26 Qu, Z.; Tian, T.; Tan, Y.; Ji, X.; Deng, G. J.; Huang, H. Redox-Neutral Ketyl Radical Coupling/Cyclization of Carbonyls with N-aryl Acrylamides through Consecutive Photoinduced Electron Transfer. Green Chem. 2022, 24, 7403–7409.
- 27 Ma, C. H.; Meng, H.; Li, J.; Yang, X. G.; Jiang, Y. Q.; Yu, B. Photocatalytic Transition-Metal-Free Direct 3-Acetalation of Quinoxaline-2(1H)- ones. Chin. J. Chem. 2022, 40, 2655–2662.
- 28 Shi, A. Z.; Sun, K.; Wu, Y. X.; Xiang, P. J.; Krylov, I. B.; Terent’ev, A. O.; Chen, X. L.; Yu, B. Oxygen-Doped Carbon Nitride for Enhanced Photocatalytic Activity in Visible-Light-Induced Decarboxylative Annulation Reactions. J. Catal. 2022, 415, 28–36.
- 29 Hu, J. Y.; Zhu, Z. Q.; Xie, Z. B.; Le, Z. G. Recent Advances in Visible-Light-Induced Decarboxylative Coupling Reactions of α-Amino Acid Derivatives. Chin. J. Org. Chem. 2022, 42, 978–1001.
- 30 Shi, T.; Liu, Y. T.; Wang, S. S.; Lv, Q. Y.; Yu, B. Recyclable Carbon Nitride Nanosheet-Photocatalyzed Aminomethylation of Imidazo[1,2-a]pyridines in Green Solvent. Chin. J. Chem. 2022, 40, 97–103.
- 31 Lu, Y. H.; Zhang, Z. T.; Wu, H. Y.; Zhou, M. H.; Song, H. Y.; Ji, H. T.; Jiang, J.; Chen, J. Y.; He, W. M. TBAI/H2O-Cooperative Electrocatalytic Decarboxylation Coupling-Annulation of Quinoxaline-2(1H)-ones with N-arylglycines. Chin. Chem. Lett. 2023, 34, 108036.
- 32 Wan, Y.; Zhu, J. J.; Yuan, Q. Y.; Wang, W.; Zhang, Y. Q. Synthesis of β-Silyl α-Amino Acids via Visible-Light-Mediated Hydrosilylation. Org. Lett. 2021, 23, 1406–1410.
- 33 Sakakibara, Y.; Murakami, K. Switchable Divergent Synthesis Using Photocatalysis. ACS Catal. 2022, 12, 1857–1878.
- 34 Zuo, Z.-W.; MacMillan, D. W. C. Decarboxylative Arylation of α-Amino Acids via Photoredox Catalysis: A One-Step Conversion of Biomass to Drug Pharmacophore. J. Am. Chem. Soc. 2014, 136, 5257−5260.




