Artificial Water Channel Promoting Depolymerization of Actin Filaments to Trigger Cancer Cell Apoptosis
Lei Zhang
Department of Chemistry, Fudan University, 220 Handan Road, Shanghai, 200433 China
Search for more papers by this authorYin-Gui Cao
State Key Laboratory of Silkworm Genome Biology, College of Sericulture, Textile, and Biomass Sciences Institution, Southwest University, Chongqing, 400715 China
Search for more papers by this authorTing Fan
ENT Institute and Otorhinolaryngology Department of Eye & ENT Hospital, Fudan University, Shanghai, 200031
Search for more papers by this authorJiatong Zhao
Fudan International School, 325 Guoquan Road, Shanghai, 200433 China
Search for more papers by this authorYong-Hong Fu
Department of Chemistry, Fudan University, 220 Handan Road, Shanghai, 200433 China
Search for more papers by this authorQi Xiao
Department of Chemistry, Fudan University, 220 Handan Road, Shanghai, 200433 China
Search for more papers by this authorZhan-Ting Li
Department of Chemistry, Fudan University, 220 Handan Road, Shanghai, 200433 China
Search for more papers by this authorCorresponding Author
Yunfeng Wang
ENT Institute and Otorhinolaryngology Department of Eye & ENT Hospital, Fudan University, Shanghai, 200031
E-mail: [email protected], [email protected], [email protected]Search for more papers by this authorCorresponding Author
Bo Xiao
State Key Laboratory of Silkworm Genome Biology, College of Sericulture, Textile, and Biomass Sciences Institution, Southwest University, Chongqing, 400715 China
E-mail: [email protected], [email protected], [email protected]Search for more papers by this authorCorresponding Author
Jun-Li Hou
Department of Chemistry, Fudan University, 220 Handan Road, Shanghai, 200433 China
E-mail: [email protected], [email protected], [email protected]Search for more papers by this authorLei Zhang
Department of Chemistry, Fudan University, 220 Handan Road, Shanghai, 200433 China
Search for more papers by this authorYin-Gui Cao
State Key Laboratory of Silkworm Genome Biology, College of Sericulture, Textile, and Biomass Sciences Institution, Southwest University, Chongqing, 400715 China
Search for more papers by this authorTing Fan
ENT Institute and Otorhinolaryngology Department of Eye & ENT Hospital, Fudan University, Shanghai, 200031
Search for more papers by this authorJiatong Zhao
Fudan International School, 325 Guoquan Road, Shanghai, 200433 China
Search for more papers by this authorYong-Hong Fu
Department of Chemistry, Fudan University, 220 Handan Road, Shanghai, 200433 China
Search for more papers by this authorQi Xiao
Department of Chemistry, Fudan University, 220 Handan Road, Shanghai, 200433 China
Search for more papers by this authorZhan-Ting Li
Department of Chemistry, Fudan University, 220 Handan Road, Shanghai, 200433 China
Search for more papers by this authorCorresponding Author
Yunfeng Wang
ENT Institute and Otorhinolaryngology Department of Eye & ENT Hospital, Fudan University, Shanghai, 200031
E-mail: [email protected], [email protected], [email protected]Search for more papers by this authorCorresponding Author
Bo Xiao
State Key Laboratory of Silkworm Genome Biology, College of Sericulture, Textile, and Biomass Sciences Institution, Southwest University, Chongqing, 400715 China
E-mail: [email protected], [email protected], [email protected]Search for more papers by this authorCorresponding Author
Jun-Li Hou
Department of Chemistry, Fudan University, 220 Handan Road, Shanghai, 200433 China
E-mail: [email protected], [email protected], [email protected]Search for more papers by this authorComprehensive Summary
Actin filaments play important physiological functions, which have become potential targets of antitumor drugs. Using chemicals to intervene their polymerization-depolymerization dynamics would generate new strategies for designing antitumor drugs. In this report, an artificial water channel appending acetazolamide moiety, a ligand that can selectively bind to carbonic anhydrase IX, has been prepared. We demonstrated that this conjugate can target colorectal cancer cells overexpressing carbonic anhydrase IX and trigger the depolymerization of actin filaments of the cancer cells by selectively mediating water transmembrane transport. Moreover, the conjugate-promoted actin depolymerization led to tumor cell apoptosis and its high antitumor activity in vitro and in vivo against colorectal cancer. The method described herein represents a new and general strategy for designing antitumor drugs by using artificial channel-mediated selective water transport to promote actin depolymerization.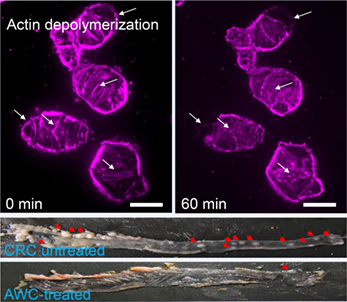
Supporting Information
| Filename | Description |
|---|---|
| cjoc202300736-sup-0001-supinfo.pdfPDF document, 2.3 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Lodish, H.; Baltimore, D.; Berk, A.; Zipursky, S. L.; Matsudaira, P.; Darnell, J. Mol. Cell Biol., 3rd edition, Scientific American Books, New York, 1995.
- 2 Janmey, P. A.; Chaponnier, C. Medical aspects of the actin cytoskeleton. Curr. Opin. Cell Biol. 1995, 7, 111–117.
- 3 Sigmond, S. H. Signal transduction and actin filament organization. Curr. Opin. Cell Biol. 1996, 8, 66–73.
- 4 Jordan, M. A.; Wilson, L. Microtubules and actin filaments: dynamic targets for cancer chemotherapy. Curr. Opin. Cell Biol. 1998, 10, 123–130.
- 5 Spector, I.; Shochet, N. R.; Blasberger, D.; Kashman, Y. Latrunculins—novel marine macrolides that disrupt microfilament organization and affect cell growth: I. Comparison with cytochalasin D. Cell Motil. Cytoskeleton 1989, 13, 127–144.
- 6 Bubb, M. R.; Senderowicz, A. M.; Sausville, E. A.; Duncan, K. L.; Korn, E. D. Jasplakinolide, a cytotoxic natural product, induces actin polymerization and competitively inhibits the binding of phalloidin to F-actin. J. Biol. Chem. 1994, 269, 14869–14871.
- 7(a) Sasaki, S.; Yui, N.; Noda, Y. Actin directly interacts with different membrane channel proteins and influences channel activities: AQP2 as a model. Biochim. Biophys. Acta Biomembr. 2014, 1838, 514–520; (b) Meli, R.; Pirozzi, C.; Pelagalli, A. New Perspectives on the Potential Role of Aquaporins (AQPs) in the Physiology of Inflammation. Front. Physiol. 2018, 9, 101–111; (c) Riethmüller, C.; Oberleithner, H.; Wilhelmi, M.; Franz, J.; Schlatter, E.; Klokkers, J.; Edemir, B. Translocation of Aquaporin-Containing Vesicles to the Plasma Membrane Is Facilitated by Actomyosin Relaxation. Biophys. J. 2008, 94, 671–678; (d) Yui, N.; Lu, H. J.; Bouley, R.; Brown, D. AQP2 is necessary for vasopressin- and forskolin-mediated filamentous actin depolymerization in renal epithelial cells. Biol. Open 2012, 1, 101–108.
- 8(a) Gourlay, C. W.; Ayscough, K. R. The actin cytoskeleton: a key regulator of apoptosis and ageing? Nat. Rev. Mol. Cell Biol. 2005, 6, 583–589; (b) Desouza, M.; Gunning, P. W.; Stehn, J. R. The actin cytoskeleton as a sensor and mediator of apoptosis. BioArchitecture 2012, 2, 75–87; (c) Thomas, S. G.; Huang, S.; Li, S.; Staiger, C. J.; Franklin-Tong, V. E. Actin depolymerization is sufficient to induce programmed cell death in self-incompatible pollen. J. Cell Biol. 2006, 174, 221–229; (d) Kulms, D.; Düssmann, H.; Pöppelmann, B.; Ständer, S.; Schwarz, A.; Schwarz, T. Apoptosis induced by disruption of the actin cytoskeleton is mediated via activation of CD95 (Fas/APO-1). Cell Death Differ. 2002, 9, 598–608.
- 9(a) Su, D.-D.; Barboiu, M. Artificial Water Channels—Progress Innovations and Prospects. CCS Chem. 2023, 5, 279–291; (b) Itoh, Y.; Chen, S.; Hirahara, R.; Konda, T.; Aoki, T.; Ueda, T.; Shimada, I.; Cannon, J. J.; Shao, C.; Shiomi, J.; Tabata, K. V.; Noji, H.; Sato, K.; Aida, T. Ultrafast water permeation through nanochannels with a densely fluorous interior surface. Science 2022, 376, 738–743; (c) Barboiu, M.; Gilles, A. From Natural to Bioassisted and Biomimetic Artificial Water Channel Systems. Acc. Chem. Res. 2013, 46, 2814–2823; (d) Hu, X.-B.; Chen, Z.; Tang, G.; Hou, J.-L.; Li, Z.-T. Single-Molecular Artificial Transmembrane Water Channels. J. Am. Chem. Soc. 2012, 134, 8384–8387; (e) Duc, Y. L.; Michau, M.; Gilles, A.; Gence, V.; Legrand, Y.-M.; van der Lee, A.; Tingry, S.; Barboiu, M. Imidazole-Quartet Water and Proton Dipolar Channels. Angew. Chem. Int. Ed. 2011, 50, 11366–11372.
- 10(a) Shen, Y. Beating natural proteins at filtering water. Science 2022, 376, 698–699; (b) Shen, Y.; Fei, F.; Zhong, Y.; Fan, C.; Sun, J.; Hu, J.; Gong, B.; Czajkowsky, D. M.; Shao, Z. Controlling Water Flow through a Synthetic Nanopore with Permeable Cations. ACS Cent. Sci. 2021, 7, 2092–2098; (c) Shen, J.; Ye, R.; Romanies, A.; Roy, A.; Chen, F.; Ren, C.; Liu, Z.; Zeng, H. Aquafoldmer-Based Aquaporin-like Synthetic Water Channel. J. Am. Chem. Soc. 2020, 142, 10050–10058; (d) Song, W.; Joshi, H.; Chowdhury, R.; Najem, J. S.; Shen, Y.-X.; Lang, C.; Henderson, C. B.; Tu, Y.-M.; Farell, M.; Pitz, M. E.; Maranas, C. D.; Cremer, P. S.; Hickey, R. J.; Sarles, S. A.; Hou, J.-L.; Aksimentiev, A.; Kumar, M. Artificial water channels enable fast and selective water permeation through water-wire networks. Nat. Nanotechnol. 2020, 15, 73–79; (e) Werber, J. R.; Osuji, C. O.; Elimelech, M. Materials for next-generation desalination and water purification membranes. Nat. Rev. Mater. 2016, 1, 16018; (f) Shen, Y.-X.; Si, W.; Erbakan, M.; Decker, K.; De Zorzi, R.; Saboe, P. O.; Kang, Y. J.; Majd, S.; Butler, P. J.; Walz, T.; Aksimentiev, A.; Hou, J.-L.; Kumar, M. Highly permeable artificial water channels that can self-assemble into two-dimensional arrays. Proc. Natl. Acad. Sci. U. S. A. 2015, 112, 9810–9815; (g) Tang, C. Y.; Zhao, Y.; Wang, R.; Helix-Nielsen, C.; Fane, A. G. Desalination by biomimetic aquaporin membranes: Review of status and prospects. Desalination 2013, 308, 34–40; (h) Kaucher, M. S.; Peterca, M.; Dulcey, A. E.; Kim, A. J.; Vinogradov, S. A.; Hammer, D. A.; Heiney, P. A.; Percec, V. Selective transport of water mediated by porous dendritic dipeptides. J. Am. Chem. Soc. 2007, 129, 11698–11699.
- 11(a) Xiao, Q.; Fan, T.; Wang, Y.; Li, Z.-T.; Hou, J.-L.; Wang, Y. Artificial Water Channel that Couples with Cell Protrusion Formation. CCS Chem. 2023, 5, 1745–1752; (b) Yan, Z.-J.; Wang, D.; Ye, Z.; Fan, T.; Wu, G.; Deng, L.; Yang, L.; Li, B.; Liu, J.; Ma, T.; Dong, C.; Li, Z.-T.; Xiao, L.; Wang, Y.; Wang, W.; Hou, J.-L. Artificial Aquaporin That Restores Wound Healing of Impaired Cells. J. Am. Chem. Soc. 2020, 142, 15638–15643.
- 12(a) Mahon, B. P.; Pinard, M. A., McKenna, R. Targeting Carbonic Anhydrase IX Activity and Expression. Molecules 2015, 20, 2323–2348; (b) Supuran, C. T. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat. Rev. Drug Discov. 2008, 7, 168–181; (c) Thiry, A.; Dogné, J. M.; Masereel, B.; Supuran, C. T. Targeting tumor-associated carbonic anhydrase IX in cancer therapy. Trends Pharmacol. Sci. 2006, 27, 566–573.
- 13(a) Supuran, C. T.; Scozzafava, A.; Casini, A. Carbonic anhydrase inhibitors. Med. Res. Rev. 2003, 23, 146–189; (b) Ahlskog, J. K. J.; Dumelin, C. E.; Trüsel, S.; Mårlind, J.; Neri, D. In vivo targeting of tumor-associated carbonic anhydrases using acetazolamide derivatives. Bioorg. Med. Chem. Lett. 2009, 19, 4851–4856; (c) Xu, Y.-S.; Wang, H.; Qiao, Z.-Y. Precise Control of Self-assembly in vivo Based on Polymer-Peptide Conjugates. Chin. J. Chem. 2022, 40, 2815–2824.
- 14(a) D'Arcy, M. S. Cell death: a review of the major forms of apoptosis, necrosis and autophagy. Cell Biol. Int. 2019, 43, 582–592; (b) Chen, Q.; Kang, J.; Fu, C. The independence of and associations among apoptosis, autophagy, and necrosis. Signal Transduct. Target. Ther. 2018, 3, 18–28; (c) Liu, L.; Kong, Y.; He, L.; Wang, X.; Wang, M.-M.; Xu, H.; Yang, C.-G.; Su, Z.; Zhao, J.; Mao, Z.-W.; Huang, Y.; Liu, H.-K. A Rhein-Based Rh(III) Arene Complex with Anti-tumor Cell Proliferative Activity Inhibits RNA Demethylase FTO. Chin. J. Chem. 2022, 40, 1156–1164.
- 15 Lukinavičius, G.; Reymond, L.; D'Este, E.; Masharina, A.; Göttfert, F.; Ta, H.; Güther, A.; Fournier, M.; Rizzo, S.; Waldmann, H.; Blaukopf, C.; Sommer, C.; Gerlich, D. W.; Arndt, H.; Hell, S. W.; Johnsson, K. Fluorogenic probes for live-cell imaging of the cytoskeleton. Nat. Methods 2014, 11, 731–733.
- 16(a) Pfaendtner, J.; De La Cruz, E. M.; Voth, G. A. Actin filament remodeling by actin depolymerization factor/cofilin. Proc. Natl. Acad. Sci. U. S. A. 2010, 107, 7299–7304; (b) Tanaka, K.; Takeda, S.; Mitsuoka, K.; Oda, T.; Kimura-Sakiyama, C.; Maéda, Y.; Narita, A. Structural basis for cofilin binding and actin filament disassembly. Nat. Commun. 2018, 9, 1860–1871; (c) Dong, W.; Ma, W.-J.; Ma, Y.-B.; Li, F.-J.; Li, T.-Z.; Wang, Y.-C.; He, X.-F.; Geng, C.-A.; Zhang, X.-M.; Chen, J.-J. Guaiane-type Sesquiterpenoid Dimers from Artemisia zhongdianensis and Antihepatoma Carcinoma Activity via the p38MAPK Pathway. Chin. J. Chem. 2023, 41, 2453–2468; (d) Qin, Y.-Y.; Li, W.-S.; Zhang, X.; Yu, Z.-X.; Li, X.-B.; Chen, H.-Y.; Lv, Y.-R.; Han, C.-R.; Chen, G.-Y. Meroterpenoids Possessing Diverse Rearranged Skeletons with Anti-inflammatory Activity from the Mangrove-Derived Fungus Penicillium sp. HLLG-122. Chin. J. Chem. 2023, 41, 2507–2517.
- 17(a) Witke, W.; Sutherland, J. D.; Sharpe, A.; Arai, M.; Kwiatkowski, D. J. Profilin I is essential for cell survival and cell division in early mouse development. Proc. Natl. Acad. Sci. U. S. A. 2001, 98, 3832–3836; (b) Krishnan, K.; Moens, P. D. J. Structure and functions of profilins. Biophys. Rev. 2009, 1, 71–81.
- 18(a) Ma, L.; Chen, Q.; Ma, P.; Han, M. K.; Xu, Z.; Kang, Y.; Xiao, B.; Merlin, D. iRGD-functionalized PEGylated nanoparticles for enhanced colon tumor accumulation and targeted drug delivery. Nanomedicine (Lond) 2017, 12, 1991–2006; (b) Xiao, B.; Viennois, E.; Chen, Q.; Wang, L.; Han, M. K.; Zhang, Y.; Zhang, Z.; Kang, Y.; Wan, Y.; Merlin, D. Silencing of Intestinal Glycoprotein CD98 by Orally Targeted Nanoparticles Enhances Chemosensitization of Colon Cancer. ACS Nano 2018, 12, 5253–5265.
- 19(a) Ma, Y.; Duan, L.; Sun, J.; Gou, S.; Chen, F.; Liang, Y.; Dai, F.; Xiao, B. Oral nanotherapeutics based on Antheraea pernyi silk fibroin for synergistic treatment of ulcerative colitis. Biomaterials 2022, 282, 21410; (b) Liu, S.; Cao, Y.; Ma, L.; Sun, J.; Ramos-Mucci, L.; Ma, Y.; Yang, X.; Zhu, Z.; Zhang, J.; Xiao, B. Oral antimicrobial peptide-EGCG nanomedicines for synergistic treatment of ulcerative colitis. J. Controlled Release 2022, 347, 544–560; (c) Xiao, B.; Laroui, H.; Viennois, E.; Ayyadurai, S.; Charania, M. A.; Zhang, Y.; Zhang, Z.; Baker, M. T.; Zhang, B.; Gewirtz, A. T.; Merlin, D. Nanoparticles with surface antibody against CD98 and carrying CD98 small interfering RNA reduce colitis in mice. Gastroenterology 2014, 146, 1289–1300.
- 20(a) Fung, K. Y.; Putoczki, T. In Vivo Models of Inflammatory Bowel Disease and Colitis-Associated Cancer. Methods Mol. Biol. 2018, 1725, 3–13; (b) Becker, C.; Fantini, M. C.; Wirtz, S.; Nikolaev, A.; Kiesslich, R.; Lehr, H. A.; Galle, P. R.; Neurath, M. F. In vivo imaging of colitis and colon cancer development in mice using high resolution chromoendoscopy. Gut 2005, 54, 950–954.
- 21 Kim, Y.; Wu, A. G.; Jaja-Chimedza, A.; Graf, B. L.; Waterman, C.; Verzi, M. P.; Raskin, I. Isothiocyanate-enriched moringa seed extract alleviates ulcerative colitis symptoms in mice. PLoS One 2017, 12, e184709.
- 22(a) O'Connell, K. E.; Mikkola, A. M.; Stepanek, A. M.; Vernet, A.; Hall, C. D.; Sun, C. C., Yildirim, E.; Staropoli, J. F.; Lee, J. T.; Brown, D. E. Practical murine hematopathology: a comparative review and implications for research. Comp. Med. 2015, 65, 96–113; (b) Wu, Y.; Sun, L.; Chen, X.; Liu, J.; Ouyang, J.; Zhang, X.; Guo, Y.; Chen, Y.; Yuan, W.; Wang, D.; He, T.; Zeng, F.; Chen, H.; Wu, S.; Zhao, Y. Cucurbit[8]uril- based water-dispersible assemblies with enhanced optoacoustic performance for multispectral optoacoustic imaging. Nat. Commun. 2023, 14, 3918–3935; (c) Wang, J.; Wu, Y.; Luo, D.; Zhuang, C.; Ning, N.; Zhang, Y.; He, Z.; Gao, J.; Hong, Z.; Xv, X.; Zhang, W.; Li, T.; Miao, Z.; Wang, H. Discovery of a Potent Botulinum Neurotoxin A Inhibitor ZM299 with Effective Protections in Botulism Mice. Chin. J. Chem. 2022, 40, 357–364.




