Tailoring the Properties of Polyhydroxyalkanoates from Plastics to Elastomers via Stereoselective Copolymerizations of rac-β-Butyrolactone and β-Propiolactone
Haoran Zhang
Beijing National Laboratory for Molecular Sciences, Key Laboratory of Polymer Chemistry and Physics of Ministry of Education, Center for Soft Matter Science and Engineering, College of Chemistry and Molecular Engineering, Peking University, Beijing, 100871 China
Search for more papers by this authorJiaojiao Qin
Beijing National Laboratory for Molecular Sciences, Key Laboratory of Polymer Chemistry and Physics of Ministry of Education, Center for Soft Matter Science and Engineering, College of Chemistry and Molecular Engineering, Peking University, Beijing, 100871 China
Search for more papers by this authorCorresponding Author
Xiaoyan Tang
Beijing National Laboratory for Molecular Sciences, Key Laboratory of Polymer Chemistry and Physics of Ministry of Education, Center for Soft Matter Science and Engineering, College of Chemistry and Molecular Engineering, Peking University, Beijing, 100871 China
*E-mail: [email protected]Search for more papers by this authorHaoran Zhang
Beijing National Laboratory for Molecular Sciences, Key Laboratory of Polymer Chemistry and Physics of Ministry of Education, Center for Soft Matter Science and Engineering, College of Chemistry and Molecular Engineering, Peking University, Beijing, 100871 China
Search for more papers by this authorJiaojiao Qin
Beijing National Laboratory for Molecular Sciences, Key Laboratory of Polymer Chemistry and Physics of Ministry of Education, Center for Soft Matter Science and Engineering, College of Chemistry and Molecular Engineering, Peking University, Beijing, 100871 China
Search for more papers by this authorCorresponding Author
Xiaoyan Tang
Beijing National Laboratory for Molecular Sciences, Key Laboratory of Polymer Chemistry and Physics of Ministry of Education, Center for Soft Matter Science and Engineering, College of Chemistry and Molecular Engineering, Peking University, Beijing, 100871 China
*E-mail: [email protected]Search for more papers by this authorComprehensive Summary
Poly(3-hydroxybutyrate), a crucial member of the large biodegradable polyhydroxyalkanoate family, suffers from its brittleness. To enhance its performance, we employed a straightforward approach involving the ring-opening copolymerization of racemic-β-butyrolactone (rac-β-BL) and β-propiolactone (β-PL) using the syndio-selective amino-alkoxy-bis(phenolate)-yttrium complex as a catalyst, thanks to the excellent ductility of poly(3-hydroxypropionate). Control over the rac-β-BL/β-PL feeding ratios and polymerization time yielded random or block copolymers with tunable thermal and mechanical properties comparable to traditional fossil-based plastics. Furthermore, we achieved one-pot synthesis of hard-soft-hard triblock copolymers by exploiting monomers’ different copolymerization rates and a bifunctional initiator, thus transforming polyhydroxyalkanoates from hard and tough plastics to soft and ductile thermoplastic elastomers.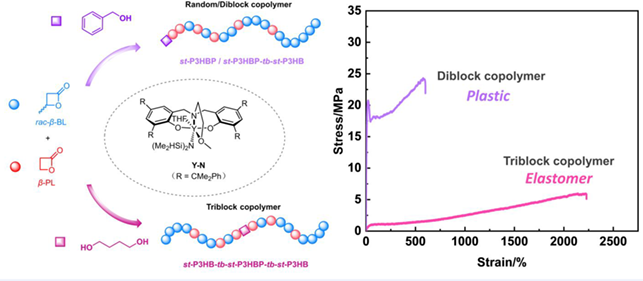
Supporting Information
| Filename | Description |
|---|---|
| cjoc202300684-sup-0001-supinfo.pdfPDF document, 6.7 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Gil-Castell, O.; Andres-Puche, R.; Dominguez, E.; Verdejo, E.; Monreal, L.; Ribes-Greus, A. Influence of substrate and temperature on the biodegradation of polyester-based materials: Polylactide and poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) as model cases. Polym. Degrad. Stab. 2020, 180, 109288.
- 2Foundation, E. M. The New Plastics Economy: Catalysing Action, 2017, https://ellenmacarthurfoundation.org/the-new-plastics-economy-catalysing-action.
- 3 Anjum, A.; Zuber, M.; Zia, K. M.; Noreen, A.; Anjum, M. N.; Tabasum, S. Microbial production of polyhydroxyalkanoates (PHAs) and its copolymers: A review of recent advancements. Int. J. Biol. Macromol. 2016, 89, 161–174.
- 4 Muhammadi; Shabina; Afzal, M.; Hameed, S. Bacterial polyhydroxyalkanoates-eco-friendly next generation plastic: Production, biocompatibility, biodegradation, physical properties and applications. Green Chem. Lett. Rev. 2015, 8, 56–77.
- 5 Laycock, B.; Halley, P.; Pratt, S.; Werker, A.; Lant, P. The chemomechanical properties of microbial polyhydroxyalkanoates. Prog. Polym. Sci. 2014, 39, 397–442.
- 6
Yves, P.; Christiane, N.; Chris, S. Production of Polyhydroxyalkanoates, a Family of Biodegradable Plastics and Elastomers, in Bacteria and Plants. Nat. Biotechnol. 1995, 13, 142–150.
10.1038/nbt0295-142 Google Scholar
- 7 Andreessen, B.; Steinbuchel, A. Biosynthesis and biodegradation of 3-hydroxypropionate-containing polyesters. Appl. Environ. Microbiol. 2010, 76, 4919–4925.
- 8 Cao, A.; Kasuya, K.-i.; Abe, H.; Doi, Y.; Inoue, Y. Studies on comonomer compositional distribution of the bacterial poly(3-hydroxybutyric acid-co-3-hydroxypropionic acid)s and crystal and thermal characteristics of their fractionated component copolyesters. Polymer 1998, 39, 4801–4816.
- 9 Sudesh, K.; Abe, H.; Doi, Y. Synthesis, structure and properties of polyhydroxyalkanoates:biological polyesters. Prog. Polym. Sci. 2000, 25, 1503–1555.
- 10 Steinbiichel, A.; Valentin, H. E. Diversity of bacterial polyhydroxyalkanoic acids. FEMS Microbiol. Lett. 1995, 128, 219–228.
- 11 Weng, C.; Tang, X. Circular Polymers from Ring-Opening Polymerization of Five-Membered (Thio)lactones and Derivatives. Chin. J. Chem. 2023, 41, 1603–1607.
- 12 Sangroniz, A.; Zhu, J.-B.; Tang, X.; Etxeberria, A.; Chen, E. Y.-X.; Sardon, H. Packaging materials with desired mechanical and barrier properties and full chemical recyclability. Nat. Commun. 2019, 10, 3359.
- 13 Worch, J. C.; Prydderch, H.; Jimaja, S.; Bexis, P.; Becker, M. L.; Dove, A. P. Stereochemical enhancement of polymer properties. Nat. Rev. Chem. 2019, 3, 514–535.
- 14 Hori, Y.; Suzuki, M.; Yamaguchi, A.; Nishishita, T. Ring-opening polymerization of optically active β-butyrolactone using distannoxane catalysts: synthesis of high-molecular-weight poly(3-hydroxybutyrate). Macromolecules 1993, 26, 5533–5534.
- 15 Liao, X.; Su, Y.; Tang, X. Stereoselective synthesis of biodegradable polymers by salen-type metal catalysts. Sci. China Chem. 2022, 65, 2096–2121.
- 16 Amgoune, A.; Thomas, C. M.; Ilinca, S.; Roisnel, T.; Carpentier, J.-F. Highly active, productive, and syndiospecific yttrium initiators for the polymerization of racemic beta-butyrolactone. Angew. Chem. Int. Ed. 2006, 45, 2782–2784.
- 17 Ajellal, N.; Bouyahyi, M.; Amgoune, A.; Thomas, C. M.; Bondon, A.; Pillin, I.; Grohens, Y.; Carpentier, J.-F. Syndiotactic-Enriched Poly(3-hydroxybutyrate)s via Stereoselective Ring-Opening Polymerization of Racemic-Butyrolactone with Discrete Yttrium Catalysts. Macromolecules 2009, 42, 987–993.
- 18 Grunova, E.; Kirillov, E.; Roisnel, T.; Carpentier, J.-F. Group 3 metal complexes supported by tridentate pyridine- and thiophene-linked bis(naphtholate) ligands: synthesis, structure, and use in stereoselective ring-opening polymerization of racemic lactide and β-butyrolactone. Dalton Trans. 2010, 39, 6739–6752.
- 19 Bouyahyi, M.; Ajellal, N.; Kirillov, E.; Thomas, C. M.; Carpentier, J.-F. Exploring electronic versus steric effects in stereoselective ring- opening polymerization of lactide and beta-butyrolactone with amino-alkoxy-bis(phenolate)-yttrium complexes. Chemistry 2011, 17, 1872–1883.
- 20 Bloembergen, S.; Holden, D. A. Stereoregularity in Synthetic β-Hydroxybutyrate and β-Hydroxyvalerate Homopolyesters. Macromolecules 1989, 22, 1656–1663.
- 21 Wu, B.; Lenz, R. W. Stereoregular Polymerization of [R,S]-3-Butyrolactone Catalyzed by Alumoxane-Monomer Adducts. Macromolecules 1998, 31, 3473–3477.
- 22 Bruckmoser, J.; Pongratz, S.; Stieglitz, L.; Rieger, B. Highly Isoselective Ring-Opening Polymerization of rac-beta-Butyrolactone: Access to Synthetic Poly(3-hydroxybutyrate) with Polyolefin-like Material Properties. J. Am. Chem. Soc. 2023, 145, 11494–11498.
- 23 Huang, H.-Y.; Xiong, W.; Huang, Y.-T.; Li, K.; Cai, Z.; Zhu, J.-B. Spiro-salen catalysts enable the chemical synthesis of stereoregular polyhydroxyalkanoates. Nat. Catal. 2023, 6, 720–728.
- 24 Yang, L.; Zhang, Y.-Y.; Yang, G.-W.; Xie, R.; Wu, G.-P. Controlled Ring-Opening Polymerization of β-Butyrolactone Via Bifunctional Organoboron Catalysts. Macromolecules 2021, 54, 5509–5517.
- 25 Tang, X.; Chen, E. Y.-X. Chemical synthesis of perfectly isotactic and high melting bacterial poly(3-hydroxybutyrate) from bio-sourced racemic cyclic diolide. Nat. Commun. 2018, 9, 2345.
- 26 Tang, X.; Westlie, A. H.; Watson, E. M.; Chen, E. Y.-X. Stereosequenced crystalline polyhydroxyalkanoates from diastereomeric monomer mixtures. Science 2019, 366, 754–758.
- 27 Moore, T.; Adhikari, R.; Gunatillake, P. Chemosynthesis of bioresorbable poly(gamma-butyrolactone) by ring-opening polymerisation: a review. Biomaterials 2005, 26, 3771–3782.
- 28 Ajellal, N.; Thomas, C. M.; Carpentier, J.-F. Functional syndiotactic poly(β-hydroxyalkanoate)s via stereoselective ring-opening copolymerization of rac-β-butyrolactone and rac-allyl-β-butyrolactone. J. Polym. Sci. A Polym. Chem. 2009, 47, 3177–3189.
- 29 Kiriratnikom, J.; Robert, C.; Guerineau, V.; Venditto, V.; Thomas, C. M. Stereoselective Ring-Opening (Co)polymerization of beta-Butyrolactone and epsilon-Decalactone Using an Yttrium Bis(phenolate) Catalytic System. Front. Chem. 2019, 7, 301.
- 30 Adams, F.; Pehl, T. M.; Kränzlein, M.; Kernbichl, S. A.; Kang, J.-J.; Papadakis, C. M.; Rieger, B. (Co)polymerization of (−)-menthide and β-butyrolactone with yttrium-bis(phenolates): tuning material properties of sustainable polyesters. Polym. Chem. 2020, 11, 4426–4437.
- 31 Tang, X.; Westlie, A. H.; Caporaso, L.; Cavallo, L.; Falivene, L.; Chen, E. Y.-X. Biodegradable Polyhydroxyalkanoates by Stereoselective Copolymerization of Racemic Diolides: Stereocontrol and Polyolefin-Like Properties. Angew. Chem. Int. Ed. 2020, 59, 7881–7890.
- 32 Li, W.-B.; Ren, B.-H.; Gu, G.-G.; Lu, X.-B. Controlled Random Terpolymerization of β-Propiolactone, Epoxides, and CO2 via Regioselective Lactone Ring Opening. CCS Chem. 2022, 4, 344–355.
- 33 Yang, G. W.; Zhang, Y. Y.; Wu, G. P. Modular Organoboron Catalysts Enable Transformations with Unprecedented Reactivity. Acc. Chem. Res. 2021, 54, 4434–4448.
- 34 Platel, R. H.; Hurst, A. R. Precise Microstructure Control in Poly(hydroxybutyrate-co-lactic Acid) Copolymers Prepared by an Yttrium Amine Bis(phenolate) Complex. Macromolecules 2020, 53, 10773–10784.
- 35 Yang, X.; Fan, H.-Z.; Cai, Z.; Zhang, Q.; Zhu, J.-B. Ring-Opening Polymerization of a Benzyl-Protected Cyclic Ester towards Functional Aliphatic Polyester. Chin. J. Chem. 2022, 40, 2973–2980.
- 36 Tang, X.; Shi, C.; Zhang, Z.; Chen, E. Y.-X. Toughening Biodegradable Isotactic Poly(3-hydroxybutyrate) via Stereoselective Copolymerization of a Diolide and Lactones. Macromolecules 2021, 54, 9401–9409.
- 37 Tang, X.; Shi, C.; Zhang, Z.; Chen, E. Y.-X. Crystalline aliphatic polyesters from eight-membered cyclic (di)esters. J. Polym. Sci. 2022, 60, 3478–3488.
- 38 Fagerland, J.; Finne-Wistrand, A.; Pappalardo, D. Modulating the thermal properties of poly(hydroxybutyrate) by the copolymerization of rac-β-butyrolactone with lactide. New J. Chem. 2016, 40, 7671–7679.
- 39 Tian, T.; Feng, C.; Wang, Y.; Zhu, X.; Yuan, D.; Yao, Y. Synthesis of N-Methyl-o-phenylenediamine-Bridged Bis(phenolato) Lanthanide Alkoxides and Their Catalytic Performance for the (Co)Polymerization of rac-Butyrolactone and l-Lactide. Inorg. Chem. 2022, 61, 9918–9929.
- 40 Andreessen, B.; Taylor, N.; Steinbuchel, A. Poly(3-hydroxypropionate): a promising alternative to fossil fuel-based materials. Appl. Environ. Microbiol. 2014, 80, 6574–6582.
- 41 Linares-Pasten, J. A.; Sabet-Azad, R.; Pessina, L.; Sardari, R. R.; Ibrahim, M. H.; Hatti-Kaul, R. Efficient poly(3-hydroxypropionate) production from glycerol using Lactobacillus reuteri and recombinant Escherichia coli harboring L. reuteri propionaldehyde dehydrogenase and Chromobacterium sp. PHA synthase genes. Bioresour. Technol. 2015, 180, 172–176.
- 42 Aduhene, A. G.; Cui, H.; Yang, H.; Liu, C.; Sui, G.; Liu, C. Poly(3-hydroxypropionate): Biosynthesis Pathways and Malonyl-CoA Biosensor Material Properties. Front. Bioeng. Biotechnol. 2021, 9, 646995.
- 43 Yamashita, M.; Takemoto, Y.; Ihara, E.; Yasuda, H. Organolanthanide-Initiated Living Polymerizations of ε-Caprolactone, δ-Valerolactone, and β-Propiolactone. Macromolecules 1996, 29, 1798–1806.
- 44 Yasuda, T.; Aida, T.; Inoue, S. Living Polymerization of β-Lactone Catalyzed by (Tetraphenylporphinato)aluminum Chloride. Structure of the Living End. Macromolecules 1983, 16, 1792–1796.
- 45 Zhang, D.; Hillmyer, M. A.; Tolman, W. B. A New Synthetic Route to Poly[3-hydroxypropionic acid] (P[3-HP]): Ring-Opening Polymerization of 3-HP Macrocyclic Esters. Macromolecules 2004, 37, 8198–8200.
- 46 Allmendinger, M.; Eberhardt, R.; Luinstra, G.; Rieger, B. The Cobalt-Catalyzed Alternating Copolymerization of Epoxides and Carbon Monoxide: A Novel Approach to Polyesters. J. Am. Chem. Soc. 2002, 124, 5646–5647.
- 47 Dunn, E. W.; Lamb, J. R.; LaPointe, A. M.; Coates, G. W. Carbonylation of Ethylene Oxide to β-Propiolactone: A Facile Route to Poly(3-hydroxypropionate) and Acrylic Acid. ACS Catal. 2016, 6, 8219–8223.
- 48 Zhou, Q.; Shi, Z. Y.; Meng, D. C.; Wu, Q.; Chen, J. C.; Chen, G. Q. Production of 3-hydroxypropionate homopolymer and poly(3-hydroxypropionate-co-4-hydroxybutyrate) copolymer by recombinant Escherichia coli. Metab. Eng. 2011, 13, 777–785.
- 49 Fukui, T.; Suzuki, M.; Tsuge, T.; Nakamura, S. Microbial Synthesis of Poly((R)-3-hydroxybutyrate-co-3-hydroxypropionate) from Unrelated Carbon Sources by Engineered Cupriavidus necator. Biomacromolecules 2009, 10, 700–706.
- 50 Wang, Q.; Yang, P.; Xian, M.; Yang, Y.; Liu, C.; Xue, Y.; Zhao, G. Biosynthesis of poly(3-hydroxypropionate-co-3-hydroxybutyrate) with fully controllable structures from glycerol. Bioresour. Technol. 2013, 142, 741–744.
- 51
Kobayashi, T.; Yamaguchi, A.; Hagiwara, T.; Hori, Y. Synthesis of poly(3-hydroxyalkanoate)s by ring-opening copolymerization of (R)-β-butyrolactone with other four-membered lactones using a distannoxane complex as a catalyst. Polymer 1995, 36, 4704–4710.
10.1016/0032-3861(95)96839-Z Google Scholar
- 52 Catchpole, P.; Platel, R. H. Copolymerisation of β-butyrolactone and γ-butyrolactone using yttrium amine bis(phenolate) catalysts. Polym. Int. 2022, 71, 1409–1417.
- 53 Pietrangelo, A.; López-Barrón, C. R.; DeRocco, M. T.; Kang, S.; Mattler, S. J.; Wright, P. J. Tuning the Morphology, Thermal Behavior, and Toughness of Poly(β-butyrolactone-co-β-valerolactone) Thermoplastics. Macromolecules 2023, 56, 5588–5598.
- 54 Fineman, M.; Ross, S. D. Linear method for determining monomer reactivity ratios in copolymerization. J. Polym. Sci. 1950, 5, 259–262.
- 55
Nakamura, S.; Kunioka, M.; Doi, Y. Biosynthesis and Characterization of Bacterial Poly(3-Hydroxybutyrate-co-3-hydroxypropionate). J. Macromol. Sci. A Chem. 1991, 28, 15–24.
10.1080/00222339108054378 Google Scholar
- 56 Shimamura, E.; Scandola, M.; Doi, Y. Microbial Synthesis and Characterization of Poly(3-hydroxybutyrate-co-3-hydroxypropionate). Macromolecules 1994, 27, 4429–4435.
- 57 Hiki, S.; Taniguchi, I.; Miyamoto, M.; Kimura, Y. Poly([R]-3-hydroxybutyrate-co-glycolate): A Novel PHB Derivative Chemically Synthesized by Copolymerization of a New Cyclic Diester Monomer [R]-4-Methyl-1,5-dioxepane-2,6-dione. Macromolecules 2002, 35, 2423–2425.
- 58 Meng, D. C.; Shi, Z. Y.; Wu, L. P.; Zhou, Q.; Wu, Q.; Chen, J. C.; Chen, G. Q. Production and characterization of poly(3-hydroxypropionate- co-4-hydroxybutyrate) with fully controllable structures by recombinant Escherichia coli containing an engineered pathway. Metab. Eng. 2012, 14, 317–324.
- 59 Spyros, A.; Argyopoulos, D. S.; Marchessault, R. H. A Study of Poly(hydroxyalkanoate)s by Quantitative 31P NMR Spectroscopy: Molecular Weight and Chain Cleavage. Macromolecules 1997, 30, 327–329.




