Ru(II)-Catalyzed ortho C—H Allylation of N-Aryl-7-azaindoles with 2-Methylidene Cyclic Carbonate
Jing Zhang
State Key Laboratory Base of Eco-Chemical Engineering, College of Chemical Engineering, Qingdao University of Science and Technology, Qingdao, Shandong, 266042 China
Search for more papers by this authorQuan-Jian Luo
State Key Laboratory Base of Eco-Chemical Engineering, College of Chemical Engineering, Qingdao University of Science and Technology, Qingdao, Shandong, 266042 China
Search for more papers by this authorHan-Chi Wang
State Key Laboratory Base of Eco-Chemical Engineering, College of Chemical Engineering, Qingdao University of Science and Technology, Qingdao, Shandong, 266042 China
Search for more papers by this authorJin-Heng Li
State Key Laboratory Base of Eco-Chemical Engineering, College of Chemical Engineering, Qingdao University of Science and Technology, Qingdao, Shandong, 266042 China
State Key Laboratory of Applied Organic Chemistry, Lanzhou University, Lanzhou, Gansu, 730000 China
School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, Henan, 453007 China
Search for more papers by this authorCorresponding Author
Bo Sun
State Key Laboratory Base of Eco-Chemical Engineering, College of Chemical Engineering, Qingdao University of Science and Technology, Qingdao, Shandong, 266042 China
E-mail: [email protected]Search for more papers by this authorJing Zhang
State Key Laboratory Base of Eco-Chemical Engineering, College of Chemical Engineering, Qingdao University of Science and Technology, Qingdao, Shandong, 266042 China
Search for more papers by this authorQuan-Jian Luo
State Key Laboratory Base of Eco-Chemical Engineering, College of Chemical Engineering, Qingdao University of Science and Technology, Qingdao, Shandong, 266042 China
Search for more papers by this authorHan-Chi Wang
State Key Laboratory Base of Eco-Chemical Engineering, College of Chemical Engineering, Qingdao University of Science and Technology, Qingdao, Shandong, 266042 China
Search for more papers by this authorJin-Heng Li
State Key Laboratory Base of Eco-Chemical Engineering, College of Chemical Engineering, Qingdao University of Science and Technology, Qingdao, Shandong, 266042 China
State Key Laboratory of Applied Organic Chemistry, Lanzhou University, Lanzhou, Gansu, 730000 China
School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, Henan, 453007 China
Search for more papers by this authorCorresponding Author
Bo Sun
State Key Laboratory Base of Eco-Chemical Engineering, College of Chemical Engineering, Qingdao University of Science and Technology, Qingdao, Shandong, 266042 China
E-mail: [email protected]Search for more papers by this authorComprehensive Summary
A Ru(II)-catalyzed ortho allylation reaction of N-aryl-7-azaindole with readily available 2-methylidene cyclic carbonate has been developed. This reaction is an effective pathway for synthesizing 7-azaindole derivatives with a wide scope of substrates and high yields. In addition, the method can be extended to the allylation of other heterocyclic compounds and several cyclic carbonates, highlighting the practicality of this strategy for synthesis.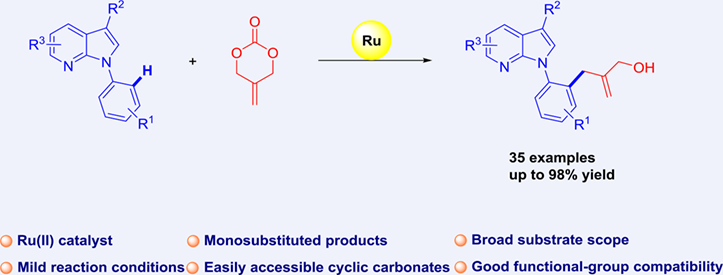
Supporting Information
| Filename | Description |
|---|---|
| cjoc202300627-sup-0001-supinfo.pdfPDF document, 4.4 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Marminon, C.; Pierré, A.; Pfeiffer, B.; Pérez, V.; Léonce, S.; Joubert, A.; Bailly, C.; Renard, P.; Hickman, J.; Prudhomme, M. Syntheses and Antiproliferative Activities of 7-Azarebeccamycin Analogues Bearing One 7-Azaindole Moiety. J. Med. Chem. 2003, 46, 609–622; (b) Hong, S.; Kim, J.; Seo, J. H.; Jung, K. H.; Hong, S. S.; Hong, S. Design, Synthesis, and Evaluation of 3,5-Disubstituted 7-Azaindoles as Trk Inhibitors with Anticancer and Antiangiogenic Activities. J. Med. Chem. 2012, 55, 5337–5349; (c) Attila Paczal, A.; Balázs Bálint, B.; Wéber, C.; Zoltán, B. Szabó, Z. B.; Ondi, L.; Theret, I.; Ceuninck, F. D.; Bernard, C.; Ktorza, A.; Francoise Perron-Sierra, F.; Kotschy, A. Structure-Activity Relationship of Azaindole-Based Glucokinase Activators. J. Med. Chem. 2016, 59, 687–706.
- 2(a) Smirnov, A. V.; English, D. S.; Rich, R. L.; Lane, R. J.; Teyton, L.; Schwabacher, A. W.; Luo, S.; Thornburg, R. W.; Petrich, J. W. Photophysics and Biological Applications of 7-Azaindole and Its Analogs. J. Phys. Chem. B 1997, 101, 2758–2769;
(b) Wu, Q.; Esteghamatian, M.; Hu, N.; Popovic, Z.; Enright, G.; Breeze, S. R.; Wang, S. Isomerism and Blue Electroluminescence of a Novel Organoboron Compound: BIII2(O)(7-azain)2Ph2. Angew. Chem. Int. Ed. 1999, 38, 985–988.
10.1002/(SICI)1521-3773(19990401)38:7<985::AID-ANIE985>3.0.CO;2-C CAS PubMed Web of Science® Google Scholar
- 3(a) Mandal, R.; Garai, B.; Sundararaju, B. Weak-Coordination in C-H Bond Functionalizations Catalyzed by 3d Metals. ACS Catal. 2022, 12, 3452–3506; (b) Gandeepan, P.; Müller, T.; Zell, D.; Cera, G.; Warratz, S.; Ackermann, L. 3d Transition Metals for C-H Activation. Chem. Rev. 2019, 119, 2192–2452; (c) Sambiagio, C.; Schonbauer, D.; Blieck, R.; Dao-Huy, T.; Pototschnig, G.; Schaaf, P.; Wiesinger, T.; Zia, M. F.; Wencel-Delord, J.; Besset, T.; Maes, B.; Schnürch, M. A Comprehensive Overview of Directing Groups Applied in Metal-Catalysed C-H Functionalisation Chemistry. Chem. Soc. Rev. 2018, 47, 6603–6743; (d) Ping, L.; Chung, D. S.; Bouffard, J.; Lee, S. Transition Metal-Catalyzed Site and Regio-Divergent C-H Bond Functionalization. Chem. Soc. Rev. 2017, 46, 4299–4328.
- 4(a) Sun, J.; Liu, M.; Zhang, J.; Dong, L. Cobalt(III)-Catalyzed C-H Amidation of 7-Azaindoles with Dioxazolones: Synthesis of 7-Azaindole Amidated Derivatives. J. Org. Chem. 2018, 83, 10555−10563; (b) Kumar, M.; Rastogi, A.; Raziullah; Ahmad, A.; Gangwar, M. K.; Koley, D. Cu(II)-Catalyzed, Site Selective Sulfoximination to Indole and Indolines via Dual C-H/N-H Activation. Org. Lett. 2022, 24, 8729−8734; (c) Banerjee, S.; Mishra, M.; Punniyamurthy, T. Copper-Catalyzed C7-Selective C-H/N-H Cross-Dehydrogenative Coupling of Indolines with Sulfoximines. Org. Lett. 2022, 24, 7997−8001; (d) Pan, C.; Wang, Y.; Wu, C.; Yu, J. T. Iridium-Catalyzed C-H Phosphoramidation of N-Aryl-7-Azaindoles with Phosphoryl Azides. Org. Biomol. Chem. 2018, 16, 3711–3715; (e) Liu, S.; Zhang, Y.; Zhao, C.; Zhou, X.; Liang, J.; Zhang, P.; Jiao, L. Y.; Yang, X.; Ma, Y. N-Aroyloxycarbamates as Switchable Nitrogen and Oxygen Precursor: Ir/Cu Controlled Divergent C-H Functionalization of Heteroarenes. Org. Chem. Front. 2022, 9, 2093−2101.
- 5(a) Kumar, M.; Raziullah; Ahmad, A.; Dutta, H. S.; Khan, A. A.; Rastogi, A.; Kant, R.; Koley, D. Cu(II)-Catalyzed C-N, C-O, C-Cl, C-S, and C-Se Bond Formation via C(sp2)-H Activation Using 7-Azaindole as an Intrinsic Directing Group. J. Org. Chem. 2021, 86, 15185−15202; (b) Vats, T. K.; Mishra, A.; Deba, I. Rhodium-Catalyzed Direct and Selective ortho C-H Chalcogenation of N-(Hetero)aryl-7-Azaindoles. Adv. Synth. Catal. 2018, 360, 2291–2296; (c) Hong, X.; Tan, Q.; Liu, B.; Xu, B. Isocyanide-Induced Activation of Copper Sulfate: Direct Access to Functionalized Heteroarene Sulfonic Esters. Angew. Chem. Int. Ed. 2017, 56, 3961–3965; (d) Yu, R.; Zhang, C.; Zhou, X.; Xiong, Y.; Duan, X. Copper-Catalyzed ortho-Selective Direct Sulfenylation of N-Aryl-7- Azaindoles with Disulfides. Org. Biomol. Chem. 2021, 19, 2901–2906.
- 6(a) Tian, Y.; Kong, X.; Niu, J.; Huang, Y.; Wu, Z.; Xu, B. Rhodium-Catalyzed Regioselective C(sp2)-H Bond Activation Reactions of N-(hetero)aryl-7-Azaindoles and Cross-Coupling with α-Carbonyl Sulfoxonium Ylides. Tetrahedron Lett. 2020, 61, 151627; (b) Yu, J.; Shan, Y.; Yuan, C.; Pan, C. Iridium-Catalyzed Selective ortho C-H Carbenoid Functionalization of N-Aryl-7-Azaindoles with Diazotized Meldrum's acid. Tetrahedron Lett. 2021, 62, 152703; (c) Li, S.; Li, W.; Zhang, G.; Xia, Y.; Liu, C.; Su, F.; Zhang, X.; Dong, L. One-Pot Construction of Fused Polycyclic Heteroarenes Involving 7-Azaindoles and α,β-Unsaturated Ketones. Org. Biomol. Chem. 2016, 14, 7859–7863; (d) Liu, J.; Jiang, J.; Yang, Z.; Zeng, Q.; Zheng, J.; Zhang, S.; Zheng, L.; Zhang, S.; Liu, Z. Q. Rhodium(III)-Catalyzed Oxidative Alkylation of N-Aryl-7-Azaindoles with Cyclopropanols. Org. Biomol. Chem. 2021, 19, 993–997.
- 7(a) Li, S.; Wang, C.; Li, W.; Zhang, X.; Dong, L. Rhodium-Catalyzed Selective Oxidative Coupling of 7-Azaindoles. Tetrahedron 2016, 72, 2581–2586; (b) Li, S.; Wang, C.; Lin, H.; Zhang, X.; Dong, L. Rhodium(III)-Catalyzed C-C Coupling of 7-Azaindoles with Vinyl Acetates and Allyl Acetates. Org. Biomol. Chem. 2016, 14, 229–237; (c) Liu, B.; Li, R.; Zhan, W.; Wang, X.; Ge, Z.; Li, R. Rh(III)-Catalyzed C-H Oxidative ortho-Olefination of Arenes Using 7-Azaindole as a Directing Group and Utilization in the Construction of New Tetracyclic Heterocycles Containing a 7-Azaindole Skeleton. RSC Adv. 2016, 6, 48205–48211; (d) Li, W. H.; Wu, L.; Li, S.; Liu, C.; Zhang, G.; Dong, L. Rhodium-Catalyzed Hydrogen-Releasing ortho-Alkenylation of 7-Azaindoles. Chem. Eur. J. 2016, 22, 17926–17929.
- 8(a) Niu, R.; Zhang, J.; Zhao, R.; Luo, Q.; Li, J. H.; Sun, B. Cobalt(III)- Catalyzed Directed C-7 Selective C-H Alkynylation of Indolines with Bromoalkynes. Org. Lett. 2023, 25, 5411−5415; (b) Liu, B.; Wang, X.; Ge, Z.; Li, R. Regioselective Ir(III)-Catalyzed C-H Alkynylation Directed by 7-Azaindoles. Org. Biomol. Chem. 2016, 14, 2944–2949.
- 9(a) Li, S.; Wang, C.; Lin, H.; Zhang, X.; Dong, L. Rhodium(III)-Catalyzed Oxidative Annulation of 7-Azaindoles and Alkynes via Double C-H Activation. Org. Lett. 2015, 17, 3018−3021; (b) Li, S.; Lin, H.; Liu, C.; Xia, Y.; Zhang, X.; Dong, L. Rhodium-Catalyzed Tandem Annulation Reactions of 7-Azaindoles with Electron-Deficient Olefins via Double C-H Activation. Adv. Synth. Catal. 2016, 358, 1595–1601; (c) Li, S.; Liu, C.; Xia, Y.; Li, W.; Zhang, G.; Zhang, X.; Dong, L. A Unique Annulation of 7-Azaindoles with Alkenyl Esters to Produce π-Conjugated 7-Azaindole Derivatives. Org. Biomol. Chem. 2016, 14, 5214–5218; (d) Liu, C.; Zhang, G.; Sun, J.; Dong, L. Access to π-Conjugated Azaindole Derivatives via Rhodium(III)-Catalyzed Cascade Reaction of Azaindoles and Diazo Compounds. Org. Biomol. Chem. 2017, 15, 2902–2905.
- 10(a) Jeong, T.; Lee, S. H.; Chun, R.; Han, S.; Han, S. H.; Jeon, Y. U.; Par, J.; Yoshimitsu, T.; Mishra, N. K.; Kim, I. S. Ru(II)-Catalyzed C-H Aminocarbonylation of N-(Hetero)aryl-7-Azaindoles with Isocyanates. J. Org. Chem. 2018, 83, 4641−4649; (b) Rajput, S.; Kaur R.; Jain, N. Pd and Photoredox Dual Catalysis Assisted Decarboxylative ortho-Benzoylation of N-Phenyl-7-Azaindoles. Org. Biomol. Chem. 2022, 20, 1453–1461.
- 11(a) Mishra, A.; Vats, T. K.; Deb, I. Ruthenium-Catalyzed Direct and Selective C-H Cyanation of N-(Hetero)aryl-7-Azaindoles. J. Org. Chem. 2016, 81, 6525−6534; (b) Wei, W.; Gong, Z.; Chai, X.; Xu, Y. J.; Dong, L. Rh(III)-Catalyzed Cross Dehydrogenative Coupling of N-Phenyl Substituent with Thiophenes. Eur. J. Org. Chem. 2023, 26, e202300294.
- 12(a) Ko, N.; Min, S.; Moon, K.; Shin, H.; Kwon, N. Y.; Mishra, N. K.; Rakshit, A.; Singh, P.; Kim, I. S. Catalyst-Controlled C-H Allylation and Annulation of 2-Aryl Quinazolinones with 2-Methylidene Cyclic Carbonate. J. Org. Chem. 2023, 88, 13315−13326; (b) Zhang, S.; Liu, Y.; Zheng, Y.; Xie, H.; Chen, S.; Song, J.; Shu, B. Rhodium(III)-Catalyzed Regioselective C-H Allylation and Prenylation of Indoles at C4-Position. Adv. Synth. Catal. 2022, 364, 64–70; (c) Zhang, Y.; Li, X.; Bai, J.; Huang, Z.; Yin, M.; Sheng, J. R.; Song, Y. Rh(III)-Catalyzed C-H Allylation/Annulative Markovnikov Addition with 5-Methylene-1,3- Dioxan-2-One: Formation of Isoquinolinones Containing a C3 Quaternary Centre. Org. Chem. Front. 2021, 8, 6863–6868; (d) Cai, X.; Song, X.; Yang, X.; Zhang, X.; Fan, X. S. A Divergent Construction of Fused and Bridged Carbo-/Heterocyclic Scaffolds via Cascade Reactions of Aryl Azomethine Imines with Vinyl Cyclic Carbonates. Org. Chem. Front. 2023, 10, 1015–1021.
- 13(a) Moon, J.; Ko, N.; Jang, S.; Ghosh, P.; Kim, H. S.; Mishra, N. K.; Kim, I. S. Ruthenium(II)-Catalyzed Tandem C-H Allylation and [3+2] Dipolar Cycloaddition to Construct Bridged Tetracycles. Org. Lett. 2022, 24, 8115−8119; (b) Xie, H.; Zeng, Y. F.; Shu, B.; Liang, J. Y.; Huang, Z. J.; Chen, S. Y.; Zheng, Y. C.; Liu, Y. Z.; Zhang, S. S. Mild Synthesis of 3,4-Dihydroisoquinolin-1(2H)-ones via Rh(III)-Catalyzed Tandem C-H-Allylation/N-Alkylation Annulation with 2-Methylidenetrimethylene Carbonate. J. Org. Chem. 2021, 86, 17063−17070; (c) Ko, N.; Min, S.; Moon, K.; Shin, H.; Kwon, N. Y.; Mishra, N. K.; Rakshit, A.; Singh, P.; Kim, I. S. Catalyst-Controlled C-H Allylation and Annulation of 2-Aryl Quinazolinones with 2-Methylidene Cyclic Carbonate. J. Org. Chem. 2023, 88, 13315–13326; (d) Min, S.; Kim, T.; Jeong, T.; Yang, J.; Oh, Y.; Moon, K.; Rakshit, A.; Kim, I. S. Synthesis of 2-Formyl Carbazoles via Tandem Reaction of Indolyl Nitrones with 2-Methylidene Cyclic Carbonate. Org. Lett. 2023, 25, 4298−4302.
- 14(a) Zhang, S.; Zheng, Y.; Zhang, Z.; Chen, S.; Xie, H.; Shu, B.; Song, J.; Liu, Y.; Zeng, Y.; Zhang, L. Access to Branched Allylarenes via Rhodium(III)-Catalyzed C-H Allylation of (Hetero)arenes with 2-Methylidenetrimethylene Carbonate. Org. Lett. 2021, 23, 5719−5723; (b) Xie, H.; Liang, J.; Huang, Z.; Shu, B.; Zheng, Y.; Liu, Y.; Chen, S.; Liu, X.; Zhang, S. Rh(III)-Catalyzed Tandem C(sp2)-H Allylation/N-Alkylation Annulation of Arene Amides with 2-Alkylidenetrimethylene Carbonates. Org. Chem. Front. 2021, 8, 6585–6590.
- 15 Zhao, R.; Zhang, J.; Niu, R.; Li, J. H.; Sun, B. Ru(II)-Catalyzed C-H Alkynylation of Ferrocenes with Bromoalkynes Directed by Carboxamide Groups. Org. Chem. Front. 2023, 10, 2007–2012.
- 16(a) Sears, J. E.; Boger, D. L. Total Synthesis of Vinblastine, Related Natural Products, and Key Analogues and Development of Inspired Methodology Suitable for the Systematic Study of Their Structure-Function Properties. Acc. Chem. Res. 2015, 48, 653−662; (b) Anagnostaki, E. E.; Zografos, A. L. “Common Synthetic Scaffolds” in the Synthesis of Structurally Diverse Natural Products. Chem. Soc. Rev. 2012, 41, 5613−5625; (c) Ishikura, M.; Yamada, K.; Abe, T. Simple Indole Alkaloids and Those with a Nonrearranged Monoterpenoid Unit. Nat. Prod. Rep. 2010, 27, 1630−1680.




