Unusual Single Electron Transfer Reactions between Alkenes and Iodine Electrophiles†
Zhengzhao Lou
Key Laboratory of Organofluorine Chemistry, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Ling Ling Road, Shanghai, 200032 China
School of Physical Science and Technology, ShanghaiTech University, 100 Haike Road, Shanghai, 201210 China
Search for more papers by this authorJingyu Hu
Key Laboratory of Organofluorine Chemistry, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Ling Ling Road, Shanghai, 200032 China
Search for more papers by this authorChuanfa Ni
Key Laboratory of Organofluorine Chemistry, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Ling Ling Road, Shanghai, 200032 China
Search for more papers by this authorXiu Wang
Key Laboratory of Organofluorine Chemistry, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Ling Ling Road, Shanghai, 200032 China
Search for more papers by this authorCorresponding Author
Jinbo Hu
Key Laboratory of Organofluorine Chemistry, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Ling Ling Road, Shanghai, 200032 China
School of Physical Science and Technology, ShanghaiTech University, 100 Haike Road, Shanghai, 201210 China
E-mail: [email protected]Search for more papers by this authorZhengzhao Lou
Key Laboratory of Organofluorine Chemistry, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Ling Ling Road, Shanghai, 200032 China
School of Physical Science and Technology, ShanghaiTech University, 100 Haike Road, Shanghai, 201210 China
Search for more papers by this authorJingyu Hu
Key Laboratory of Organofluorine Chemistry, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Ling Ling Road, Shanghai, 200032 China
Search for more papers by this authorChuanfa Ni
Key Laboratory of Organofluorine Chemistry, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Ling Ling Road, Shanghai, 200032 China
Search for more papers by this authorXiu Wang
Key Laboratory of Organofluorine Chemistry, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Ling Ling Road, Shanghai, 200032 China
Search for more papers by this authorCorresponding Author
Jinbo Hu
Key Laboratory of Organofluorine Chemistry, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Ling Ling Road, Shanghai, 200032 China
School of Physical Science and Technology, ShanghaiTech University, 100 Haike Road, Shanghai, 201210 China
E-mail: [email protected]Search for more papers by this authorThese authors contributed equally to this work.
Dedicated to the Memory of Professor Xiyan Lu.
Comprehensive Summary
The electrophilic addition to an alkene with an electrophile has been widely studied and applied in organic synthesis. The organic chemistry textbook describes the classical reaction between an alkene and an iodine electrophile (such as elemental iodine and N-iodosuccinimide (NIS)) as a typical ionic reaction, in which an iodonium ion is formed and then attacked by a nucleophile. However, in this article, we report a new and unusual reaction mode between an alkene and NIS; that is, a single electron transfer (SET) process occurs between these two reactants by forming an electron-donor acceptor complex. Not only does this unusual single electron transfer reaction between an alkene and NIS add fundamentally important knowledge to organic chemistry, it also provides a valuable synthetic method as the new SET reaction mode with opposite regioselectivity as compared with the traditional ionic mode.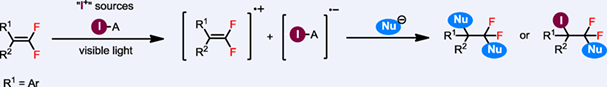
Supporting Information
| Filename | Description |
|---|---|
| cjoc202300611-sup-0001-supinfo.pdfPDF document, 10.2 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Smith, M. B. March's Advanced Organic Chemistry, Wiley, 2020; (b) Schmid, G. H.; Garrat, D. G. The Chemistry of Double-Bonded Functional Groups, John Wiley & Sons, 1989; (c) de la Mare, P. B. D.; Bolton, R. Electrophilic Additions to Unsaturated Systems, Elsevier, 1982.
- 2(a) Ashtekar, K. D.; Vetticatt, M.; Yousefi, R.; Jackson, J. E.; Borhan, B. Nucleophile-Assisted Alkene Activation: Olefins Alone Are Often Incompetent. J. Am. Chem. Soc. 2016, 138, 8114–8119; (b) Van Lommel, R.; Bock, J.; Daniliuc, C. G.; Hennecke, U.; De Proft, F. A dynamic picture of the halolactonization reaction through a combination of ab initio metadynamics and experimental investigations. Chem. Sci. 2021, 12, 7746–7757.
- 3(a) Sakakura, A.; Ishihara, K. Stereoselective Electrophilic Cyclization. Chem. Rec. 2015, 15, 728–742;
(b) Miller, E.; Kim, S.; Gibson, K.; Derrick, J. S.; Toste, F. D. Regio- and Enantioselective Bromocyclization of Difluoroalkenes as a Strategy to Access Tetrasubstituted Difluoromethylene-Containing Stereocenters. J. Am. Chem. Soc. 2020, 142, 8946–8952;
(c) Djerassi, C.; Lenk, C. T. α-Iodoketones (Part 2). Reaction of Enol Acetates with N-Iodosuccinimide. J. Am. Chem. Soc. 1953, 75, 3493–3495;
(d) Yang, H.; Zhou, Y.; Zhang, Z.; Wen, J.; Zhang, X. Iridium-Catalyzed Hydroiodination and Formal Hydroamination of Olefins with N-Iodo Reagents and Molecular Hydrogen: An Umpolung Strategy. Org. Lett. 2022, 24, 1842–1847;
(e) Zhao, S.; Li, C.; Xu, B.; Liu, H. Cp*Rh(III)-Catalyzed C—H 3,3-Difluoroallylation of Indoles and N-Iodosuccinimide-Mediated Cyclization for the Synthesis of Fluorinated 3,4-Dihydropyrimido-[1,6-a]-indol-1(2H)-one Derivatives. Chin. J. Org. Chem. 2020, 40, 1549–1562;
(f) Xiong, B.; Xu, S.; Zhu, Y.; Yao, L.; Zhou, C.; Liu, Y.; Tang, K.-W.; Wong, W.-Y. Metal-Free, N-Iodosuccinimide-Induced Regioselective Iodophosphoryloxylation of Alkenes with P(O)−OH Bonds. Chem. Eur. J. 2020, 26, 9556–9560;
(g) Jiang, Q.; Liang, Y.; Zhang, Y.; Zhao, X. Chalcogenide- Catalyzed Intermolecular Electrophilic Thio- and Halofunctionalization of gem-Difluoroalkenes: Construction of Diverse Difluoroalkyl Sulfides and Halides. Org. Lett. 2020, 22, 7581–7587;
(h) Huang, Q.; Tang, P. Silver-Mediated Intermolecular Iodotrifluoromethoxylation of Alkenes. J. Org. Chem. 2020, 85, 2512–2519;
(i) Goto, M.; Maejima, S.; Yamaguchi, E.; Itoh, A. Regioselective Carboiodination of Styrenes: N-Iodosuccinimide Affords Complete Reaction Regioselectivity. Asian J. Org. Chem. 2020, 9, 210–213;
(j) Grandclaudon, C.; Michelet, V.; Toullec, P. Y. Iodonium-Induced Cyclization of N-Allenylindoles and N-Allenylpyrroles: An Access to Iododihydropyrido[1,2-a]indoles and Dihydroindolizines. Synlett 2018, 29, 310–313;
(k) Fujita, T.; Kinoshita, R.; Takanohashi, T.; Suzuki, N.; Ichikawa, J. Ring-size-selective construction of fluorine-containing carbocycles via intramolecular iodoarylation of 1,1-difluoro-1-alkenes. Beilstein J. Org. Chem. 2017, 13, 2682–2689;
(l) Masson, G.; Lebée, C.; Blanchard, F. Highly Enantioselective Intermolecular Iodo- and Chloroamination of Enecarbamates Catalyzed by Chiral Phosphoric Acids or Calcium Phosphate Salts. Synlett 2016, 27, 559–563;
(m) Nakatsuji, H.; Sawamura, Y.; Sakakura, A.; Ishihara, K. Cooperative Activation with Chiral Nucleophilic Catalysts andN-Haloimides: Enantioselective Iodolactonization of 4-Arylmethyl-4-pentenoic Acids. Angew. Chem. Int. Ed. 2014, 53, 6974–6977;
(n) Gratia, S. S.; Vigneau, E. S.; Eltayeb, S.; Patel, K.; Meyerhoefer, T. J.; Kershaw, S.; Huang, V.; De Castro, M. A facile preparation of various N-heterocycles using amides and olefins. Tetrahedron Lett. 2014, 55, 448–452;
(o) Hajra, S.; Maji, B.; Bar, S. Samarium Triflate-Catalyzed Halogen-Promoted Friedel−Crafts Alkylation with Alkenes. Org. Lett. 2007, 9, 2783–2786;
(p) Hajra, S.; Maji, B.; Karmakar, A. Lewis acid catalyzed intramolecular halo-arylation of tethered alkenes using N-halosuccinimide (NXS) as the halogen source: a general method for the synthesis of chromanones, chromans, quinolones, tetrahydroquinolines and tetralins. Tetrahedron Lett. 2005, 46, 8599–8603;
(q) Lavilla, R.; Coll, O.; Kumar, R.; Bosch, J. Electrophilic Oxidative Additions upon 1,4-Dihydropyridines. J. Org. Chem. 1998, 63, 2728–2730;
(r) Petasis, N. A.; Zavialov, I. A. Mild conversion of alkenyl boronic acids to alkenyl halides with halosuccinimides. Tetrahedron Lett. 1996, 37, 567–570;
(s) Flekhter, O. B.; Baltina, L. A.; Vasil'eva, E. V.; Tolstikov, G. A. Stereoselective synthesis of 2,6-dideoxy-α-l-arabino-hexopyranoside of glycyrrhetic acid in the presence of iodine-containing promoters. Russ. Chem. Bull. 1996, 45, 2843–2846;
(t) Bongini, A.; Cardillo, G.; Orena, M.; Sandri, S.; Tomasini, C. Iodocyclofunctionalization of (Z)-1-trichloroacetimidoyloxyalk-2-enes and 3-trichloroacetimidoyloxyalk-1-enes. Synthesis of (±)-erythro-sphinganine triacetate and (±)-threo-sphinganine triacetate. J. Chem. Soc., Perkin Trans. 1 1986, 1339–1344.
10.1039/P19860001339 Google Scholar
- 4 Roberts, I.; Kimball, G. E. The Halogenation of Ethylenes. J. Am. Chem. Soc. 1937, 59, 947–948.
- 5(a) Olah, G. A.; Bollinger, J. M.; Brinich, J. Stable carbonium ions. LXII. Halonium ion formation via neighboring halogen participation: ethylenehalonium, propylenehalonium, and 1,2-dimethylethylenehalonium ions. J. Am. Chem. Soc. 1968, 90, 2587–2594; (b) Fukuzumi, S.; Kochi, J. K. Electrophilic additions to olefins. A new approach to unifying the mechanisms of bromination and oxymercuration. J. Am. Chem. Soc. 1981, 103, 2783–2791; (c) Prissette, J.; Seger, G.; Kochanski, E. Theoretical study of some ethylene-halogen molecule (chlorine, bromine, iodine) complexes at large and intermediate distances from ab initio calculations. J. Am. Chem. Soc. 1978, 100, 6941–6947; (d) Bellucci, G.; Chiappe, C.; Bianchini, R.; Lenoir, D.; Herges, R. Nature of the Interaction of Olefin-Bromine Complexes. Inference from (E)-2,2,5,5-Tetramethyl-3,4-diphenylhex-3-ene, the First Example of an Olefin Whose Reaction with Bromine Stops at the Stage of .pi. Complex Formation. J. Am. Chem. Soc. 1995, 117, 12001–12002; (e) Lenoir, D.; Chiappe, C. What is the Nature of the First-Formed Intermediates in the Electrophilic Halogenation of Alkenes, Alkynes, and Allenes. Chem. Eur. J. 2003, 9, 1036–1044.
- 6(a) Zhao, Y.; Gao, B.; Hu, J. From Olefination to Alkylation: In-Situ Halogenation of Julia–Kocienski Intermediates Leading to Formal Nucleophilic Iodo- and Bromodifluoromethylation of Carbonyl Compounds. J. Am. Chem. Soc. 2012, 134, 5790–5793; (b) Miao, W.; Ni, C.; Zhao, Y.; Hu, J. Nucleophilic Iododifluoromethylation of Carbonyl Compounds Using Difluoromethyl 2-Pyridyl Sulfone. Org. Lett. 2016, 18, 2766–2769.
- 7(a) Ando, O.; Nakajima, M.; Kifune, M.; Fang, H.; Tanzawa, K. Trehazolin, a slow, tight-binding inhibitor of silkworm trehalase. Biochim. Biophys. Acta (BBA) - Gen. Subj. 1995, 1244, 295–302; (b) Yang, P.-S.; Wu, H.-T.; Chung, H.-H.; Chen, C.-T.; Chi, C.-W.; Yeh, C.-H.; Cheng, J.-T. Rilmenidine improves hepatic steatosis through p38-dependent pathway to higher the expression of farnesoid X receptor. Naunyn-Schmiedeberg's Arch. Pharmacol. 2012, 385, 51–56.
- 8 Li, Q.; Woods, K. W.; Claiborne, A.; Gwaltney, I. I. S. L.; Barr, K. J.; Liu, G.; Gehrke, L.; Credo, R. B.; Hui, Y. H.; Lee, J.; Warner, R. B.; Kovar, P.; Nukkala, M. A.; Zielinski, N. A.; Tahir, S. K.; Fitzgerald, M.; Kim, K. H.; Marsh, K.; Frost, D.; Ng, S. C.; Rosenberg, S.; Sham, H. L. Synthesis and Biological Evaluation of 2-Indolyloxazolines as a New Class of Tubulin Polymerization Inhibitors. Discovery of A-289099 as an Orally Active Antitumor Agent. Bioorg. Med. Chem. Lett. 2002, 12, 465–469.
- 9(a) Sui, J.; Yang, Z.; Li, S.; Chen, X.; Zhang, X.; Shen, Q.; Jiang, H.; Li, J. Visible Light-Driven Catalyst-Free Amination of Indoles Initiated by Electron Donor-Acceptor Complexes. Chin. J. Chem. 2023, 41, 1485–1490; (b) Sun, B.; Ling, L.; Zhuang, X.; Yang, L.; Yin, J.; Jin, C. Photo-triggered Intramolecular Radical Tandem Regioselective Alkylation/Cyclization of 1,6-Dienes with Redox-Active Esters Enabled by an EDA Complex. Chin. J. Chem. 2023, 41, 37–42.
- 10 Anilkumar, R.; Burton, D. J. An efficient dehyrohalogenation method for the synthesis of α,β,β-trifluorostyrenes, α-chloro-β,β-difluorostyrenes and E-1-arylperfluoroalkenes. J. Fluorine Chem. 2005, 126, 1174–1184.




