A Pyrazine-Based 2D Conductive Metal-Organic Framework for Efficient Lithium Storage†
Xiaoxiao Sun
Tianjin Key Laboratory of Molecular Optoelectronic Science, Department of Chemistry, Tianjin University, Tianjin, 300072 China
Search for more papers by this authorCorresponding Author
Xiaoli Yan
Tianjin Key Laboratory of Molecular Optoelectronic Science, Department of Chemistry, Tianjin University, Tianjin, 300072 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorKeming Song
College of Chemistry and Green Catalysis Center, Zhengzhou University, Zhengzhou, Henan, 450001 China
Search for more papers by this authorTing Zhang
Tianjin Key Laboratory of Molecular Optoelectronic Science, Department of Chemistry, Tianjin University, Tianjin, 300072 China
Search for more papers by this authorZongfan Yang
Tianjin Key Laboratory of Molecular Optoelectronic Science, Department of Chemistry, Tianjin University, Tianjin, 300072 China
Search for more papers by this authorXi Su
Tianjin Key Laboratory of Molecular Optoelectronic Science, Department of Chemistry, Tianjin University, Tianjin, 300072 China
Search for more papers by this authorCorresponding Author
Weihua Chen
College of Chemistry and Green Catalysis Center, Zhengzhou University, Zhengzhou, Henan, 450001 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Long Chen
Tianjin Key Laboratory of Molecular Optoelectronic Science, Department of Chemistry, Tianjin University, Tianjin, 300072 China
State Key Laboratory of Supramolecular Structure and Materials, College of Chemistry, Jilin University, Changchun, Jilin, 130012 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorXiaoxiao Sun
Tianjin Key Laboratory of Molecular Optoelectronic Science, Department of Chemistry, Tianjin University, Tianjin, 300072 China
Search for more papers by this authorCorresponding Author
Xiaoli Yan
Tianjin Key Laboratory of Molecular Optoelectronic Science, Department of Chemistry, Tianjin University, Tianjin, 300072 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorKeming Song
College of Chemistry and Green Catalysis Center, Zhengzhou University, Zhengzhou, Henan, 450001 China
Search for more papers by this authorTing Zhang
Tianjin Key Laboratory of Molecular Optoelectronic Science, Department of Chemistry, Tianjin University, Tianjin, 300072 China
Search for more papers by this authorZongfan Yang
Tianjin Key Laboratory of Molecular Optoelectronic Science, Department of Chemistry, Tianjin University, Tianjin, 300072 China
Search for more papers by this authorXi Su
Tianjin Key Laboratory of Molecular Optoelectronic Science, Department of Chemistry, Tianjin University, Tianjin, 300072 China
Search for more papers by this authorCorresponding Author
Weihua Chen
College of Chemistry and Green Catalysis Center, Zhengzhou University, Zhengzhou, Henan, 450001 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Long Chen
Tianjin Key Laboratory of Molecular Optoelectronic Science, Department of Chemistry, Tianjin University, Tianjin, 300072 China
State Key Laboratory of Supramolecular Structure and Materials, College of Chemistry, Jilin University, Changchun, Jilin, 130012 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorDedicated to the Special Issue of Optoelectronic Functional Materials.
Comprehensive Summary
The merits of intrinsic electrical conductivity, high specific surface area, tunable chemical composition and tailor-made properties enable two-dimensional conductive metal-organic frameworks (2D c-MOFs) as promising next-generation electrode materials in the field of energy storage and conversion. Herein, we have designed and synthesized a novel pyrazine-based 2D c-MOF (TPQG-Cu-MOF) bearing extended π-conjugated structure and abundant redox active sites. Thanks to the excellent redox reversibility of pyrazine units and CuO2 units, as well as the insolubility of the rigid framework skeleton, TPQG-Cu-MOF as the cathode material of lithium-ion battery exhibits a reversible specific capacity (150.2 mAh·g–1 at 20 mAh·g–1), good cycling stability (capacity retention of 82.6% after 500 cycles at 1 A·g–1) and excellent rate performance. Comprehensive ex-situ spectroscopic studies revealed the reversible redox activity of pyrazine units and CuO2 units of TPQG-Cu-MOF during the Li+ insertion/extraction process. The deepening fundamental understanding of the structure-property relationship was proposed, which might pave the way for further development of efficient MOF-based energy storage devices.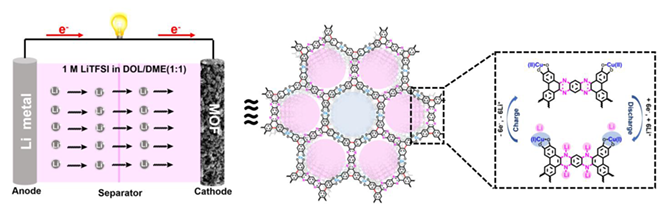
Supporting Information
| Filename | Description |
|---|---|
| cjoc202200819-sup-0001-supinfo.pdfPDF document, 1.8 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Armand, M.; Tarascon, J.-M. Building better batteries. Nature 2008, 451, 652–657.
- 2 Liu, C.; Neale, Z. G.; Cao, G. Understanding electrochemical potentials of cathode materials in rechargeable batteries. Mater. Today 2016, 19, 109–123.
- 3 Assat, G.; Tarascon, J.-M. Fundamental understanding and practical challenges of anionic redox activity in Li-ion batteries. Nat. Energy 2018, 3, 373–386.
- 4 Liu, X. L.; Li, J. L.; Li, N.; Li, B. Y.; Bu, X. H. Recent advances on metal-organic frameworks in the conversion of carbon dioxide. Chin. J. Chem. 2021, 39, 440–462.
- 5 Chen, M. P.; Lang, L.; Chen, L.; Wang, X. M.; Shi, C.; Sun, Q. W.; Xu, Y. G.; Juan, D. W.; Wang, S. Improving in vivo uranyl removal efficacy of a nano-metal organic framework by interior functionalization with 3-hydroxy-2-pyridinone. Chin. J. Chem. 2022, 40, 2054–2060.
- 6 Wang, H. G.; Wang, Y. N.; Wu, Q.; Zhu, G. S. Recent developments in electrode materials for dual-ion batteries: Potential alternatives to conventional batteries. Mater. Today 2022, 52, 269–298.
- 7 Cao, C. C.; Zhong, Y. J.; Shao, Z. P. Electrolyte engineering for safer lithium-ion btteries: A Review. Chin. J. Chem. 2023, 41, 1119–1141.
- 8 Bourgeois, J. P.; Vlad, A.; Melinte, S.; Gohy, J. F. Design of flexible and self-standing electrodes for Li-Ion batteries. Chin. J. Chem. 2017, 35, 41–47.
- 9 Yabuuchi, N.; Yoshii, K.; Myung, S. T.; Nakai, I.; Komaba, S. Detailed studies of a high-capacity electrode material for rechargeable batteries, Li2MnO3-LiCo1/3Ni1/3Mn1/3O2. J. Am. Chem. Soc. 2011, 133, 4404–4419.
- 10 Manthiram, A. A reflection on lithium-ion battery cathode chemistry. Nat. Commun. 2020, 11, 1550–1558.
- 11 Lu, Y.; Zhang, Q.; Li, L.; Niu, Z.; Chen, J. Design Strategies toward enhancing the performance of organic electrode materials in metal-ion batteries. Chem 2018, 4, 2786–2813.
- 12 Xue, L.; Li, J.; Hu, S.; Zhang, M.; Zhou, Y.; Zhan, C. Anthracene based organodisulfide positive active materials for lithium secondary battery. Elecreochem. Commun. 2003, 5, 903–906.
- 13 Wu, Y.; Zeng, R.; Nan, J.; Shu, D.; Qiu, Y.; Chou, S. Quinone electrode materials for rechargeable lithium/sodium ion batteries. Adv. Energy Mater. 2017, 7, 1700278.
- 14 Peng, C.; Ning, G.; Su, J.; Zhong, G.; Tang, W.; Tian, B.; Su, C.; Yu, D.; Zu, L.; Yang, J.; Ng, M.-F.; Hu, Y.; Yang, Y.; Armand, M.; Loh, K. Reversible multi-electron redox chemistry of π-conjugated N-containing heteroaromatic molecule-based organic cathodes. Nat. Energy 2017, 2, 17074–17083.
- 15 Li, K.; Yu, J.; Si, Z. J.; Gao, B.; Wang, H. G.; Wang, Y. H. One-dimensional π-d conjugated coordination polymer with double redox-active centers for all-organic symmetric lithium-ion batteries. Chem. Eng. J. 2022, 450, 138052.
- 16 Chen, L.; Cheng, L. Q.; Yu, J.; Chu, J.; Wang, H. G.; Cui, F. C.; Zhu, G. S. Tailored organic cathode material with multi-active site and compatible groups for stable quasi-solid-state lithium-organic batteries. Adv. Funct. Mater. 2022, 32, 2209848.
- 17 Chang, C.-H.; Li, A.-C.; Popovs, I.; Kaveevivitchai, W.; Chen, J.-L.; Chou, K.-C.; Kuo, T.-S.; Chen, T.-H. Elucidating metal and ligand redox activities of a copper-benzoquinoid coordination polymer as the cathode for lithium-ion batteries. J. Mater. Chem. A 2019, 7, 23770–23774.
- 18 Nazir, A.; Le, H. T. T.; Nguyen, A.-G.; Park, C.-J. Graphene analogue metal organic framework with superior capacity and rate capability as an anode for lithium ion batteries. Electrochim. Acta 2021, 389, 138750.
- 19 Liu, Z.; Zheng, F.; Xiong, W.; Li, X.; Yuan, A.; Pang, H. Strategies to improve electrochemical performances of pristine metal-organic frameworks-based electrodes for lithium/sodium-ion batteries. SmartMat 2021, 2, 488–518.
- 20 Wu, Z. Z.; Xie, J.; Xu, Z. C. J.; Zhang, S. Q.; Zhang, Q. C. Recent progress in metal–organic polymers as promising electrodes for lithium/sodium rechargeable batteries. J. Mater. Chem. A 2019, 7, 4259–4290.
- 21 Li, C.; Zhang, C.; Xie, J.; Wang, K. B.; Lia, J. Z.; Zhang, Q. C. Ferrocene-based metal-organic framework as a promising cathode in lithium-ion battery. Chem. Eng. J. 2021, 404, 126463.
- 22 Li, C.; Yang, H. Y.; Xie, J.; Wang, K. B.; Li, J. Z.; Zhang, Q. C. Ferrocene-based mixed-valence metal−organic framework as an efficient and stable cathode for lithium-ion-based dual-ion battery. Mater. Interfaces 2020, 12, 32719–32725.
- 23 Rojaee, R.; Shahbazian-Yassar, R. Two-dimensional materials to address the lithium battery challenges. ACS Nano 2020, 14, 2628–2658.
- 24 Duan, H.; Zhao, Z.; Lu, J.; Hu, W.; Zhang, Y.; Li, S.; Zhang, M.; Zhu, R.; Pang, H. When conductive MOFs meet MnO2: high electrochemical energy storage performance in an aqueous asymmetric supercapacitor. ACS Appl. Mater. Interfaces 2021, 13, 33083–33090.
- 25 Zhang, L.; Liu, H.; Shi, W.; Cheng, P. Synthesis strategies and potential applications of metal-organic frameworks for electrode materials for rechargeable lithium ion batteries. Coord. Chem. Rev. 2019, 388, 293–309.
- 26 Liu, Z.; Zheng, F.; Xiong, W.; Li, X.; Yuan, A., Pang, H. Strategies to improve electrochemical performances of pristine metal-organic frameworks-based electrodes for lithium/sodium-ion batteries. SmartMat 2021, 2, 488–518.
- 27 Wu, Z.; Adekoya, D.; Huang, X.; Kiefel, M. J.; Xie, J.; Xu, W.; Zhang, Q.; Zhu, D.; Zhang, S. Highly conductive two-dimensional metal-organic frameworks for resilient lithium storage with superb rate capability. ACS Nano 2020, 14, 12016–12026.
- 28 Liu, H.; Li, H.; Cheng, F.; Shi, W.; Chen, J.; Cheng, P. Enhancing the lithium storage capacities of coordination compounds for advanced lithium-ion battery anodes via a coordination chemistry approach. Inorg. Chem. 2018, 57, 10640–10648.
- 29 Slesarenko, A.; Yakuschenko, I. K.; Ramezankhani, V.; Sivasankaran, V.; Romanyuk, O.; Mumyatov, A. V.; Zhidkov, I.; Tsarev, S.; Kurmaev, E. Z.; Shestakov, A. F.; Yarmolenko, O. V.; Stevenson, K. J.; Troshin, P. A. New tetraazapentacene-based redox-active material as a promising high-capacity organic cathode for lithium and potassium batteries. J. Power Sources 2019, 435, 226724–226732.
- 30 Hong, J.; Lee, M.; Lee, B.; Seo, D. H.; Park, C. B.; Kang, K. Biologically inspired pteridine redox centres for rechargeable batteries. Nat. Commun. 2014, 5, 5335–5343.
- 31 Li, S.; Liu, Y.; Dai, L.; Li, S.; Wang, B.; Xie, J.; Li, P. A stable covalent organic framework cathode enables ultra-long cycle life for alkali and multivalent metal rechargeable batteries. Energy Stor. Mater. 2022, 48, 439–446.
- 32 Vitaku, E.; Gannett, C. N.; Carpenter, K. L.; Shen, L.; Abruna, H. D.; Dichtel, W. R. Phenazine-based covalent organic framework cathode materials with high energy and power densities. J. Am. Chem. Soc. 2020, 142, 16–20.
- 33 Qi, M.; Zhou, Y.; Lv, Y.; Chen, W.; Su, X.; Zhang, T.; Xing, G.; Xu, G.; Terasaki, O.; Chen, L. Direct Construction of 2D Conductive Metal−Organic Frameworks from a Nonplanar Ligand: In Situ Scholl Reaction and Topological Modulation. J. Am. Chem. Soc. 2023, 145, 2739–2744.
- 34 Grzybowski, M.; Skonieczny, K.; Butenschon, H.; Gryko, D. T. Comparison of oxidative aromatic coupling and the Scholl reaction. Angew. Chem. Int. Ed. 2013, 52, 9900–9930.
- 35 Rempala, P.; Kroulík, J.; King, B. T. A slippery slope: mechanistic analysis of the intramolecular Scholl reaction of hexaphenylbenzene. J. Am. Chem. Soc. 2004, 126, 15002–15003.
- 36 Sun, W.; Tang, X.; Wang, Y. Multi-metal–organic frameworks and their derived materials for Li/Na-ion batteries. Electrochem. Energy Rev. 2019, 3, 127–154.
- 37 Chu, J.; Wang, Y.; Zhong, F.; Feng, X.; Chen, W.; Ai, X.; Yang, H.; Cao, Y. Metal/covalent-organic frameworks for electrochemical energy storage applications. EcoMat 2021, 3, e12133.
- 38 Wu, Z.; Adekoya, D.; Huang, X.; Kiefel, M. J.; Xie, J.; Xu, W.; Zhang, Q.; Zhu, D.; Zhang, S. Highly conductive two-dimensional metal-organic frameworks for resilient lithium storage with superb rate capability. ACS Nano 2020, 14, 12016–12026.
- 39 Li, X.; Rong, J.; Wei, B. Electrochemical Behavior of Single-Walled Carbon Nanotube Supercapacitors Under Compressive Stress. ACS Nano 2012, 4, 6039–6049.
- 40 Selvamani, V.; Gopi, S.; Rajagopal, V.; Kathiresan, M.; Vembu, S.; Velayutham, D.; Gopukumar, S. High rate performing in situ nitrogen enriched spherical carbon particles for Li/Na-ion cells. ACS Appl. Mater. Interfaces 2017, 9, 39326–39335.
- 41 Lee, M.; Hong, J.; Seo, D.-H.; Nam, D. H.; Nam, K. T.; Kang, K.; Park, C. B. Redox cofactor from biological energy transduction as molecularly tunable energy-storage compound. Angew. Chem. Int. Ed. 2013, 52, 8322–8328.
- 42 Mao, M.; Luo, C.; Pollard, T. P.; Hou, S.; Gao, T.; Fan, X.; Cui, C.; Yue, J.; Tong, Y.; Yang, G.; Deng, T.; Zhang, M.; Ma, J.; Suo, L.; Borodin, O.; Wang, C. A pyrazine-based polymer for fast-charge batteries. Angew. Chem. Int. Ed. 2019, 58, 17820–17826.
- 43 Zhang, Z.; Yoshikawa, H.; Awaga, K. Monitoring the solid-state electrochemistry of Cu(2,7-AQDC) (AQDC = anthraquinone dicarboxylate) in a lithium battery: coexistence of metal and ligand redox activities in a metal-organic framework. J. Am. Chem. Soc. 2014, 136, 16112–16115.
Citing Literature
15 July, 2023
Pages 1691-1696




