Light-Harvesting Artificial Cells for the Generation of ATP and NADPH†
Ziwei Su
State Key Laboratory of Urban Water Resource and Environment, School of Chemistry and Chemical Engineering, Harbin Institute of Technology, 92 West Da-Zhi Street, Harbin, Heilongjiang, 150001 China
‡These authors contributed equally to this work.
†Dedicated to Professor Erkang Wang on the Occasion of His 90th Birthday.
Search for more papers by this authorXiangxiang Zhang
State Key Laboratory of Urban Water Resource and Environment, School of Chemistry and Chemical Engineering, Harbin Institute of Technology, 92 West Da-Zhi Street, Harbin, Heilongjiang, 150001 China
‡These authors contributed equally to this work.
†Dedicated to Professor Erkang Wang on the Occasion of His 90th Birthday.
Search for more papers by this authorWeichen Wang
State Key Laboratory of Urban Water Resource and Environment, School of Chemistry and Chemical Engineering, Harbin Institute of Technology, 92 West Da-Zhi Street, Harbin, Heilongjiang, 150001 China
Search for more papers by this authorMingdong Dong
Interdisciplinary Nanoscience Center (iNANO), Aarhus University, Aarhus, DK-8000 Denmark
Search for more papers by this authorCorresponding Author
Xiaojun Han
State Key Laboratory of Urban Water Resource and Environment, School of Chemistry and Chemical Engineering, Harbin Institute of Technology, 92 West Da-Zhi Street, Harbin, Heilongjiang, 150001 China
E-mail: [email protected]Search for more papers by this authorZiwei Su
State Key Laboratory of Urban Water Resource and Environment, School of Chemistry and Chemical Engineering, Harbin Institute of Technology, 92 West Da-Zhi Street, Harbin, Heilongjiang, 150001 China
‡These authors contributed equally to this work.
†Dedicated to Professor Erkang Wang on the Occasion of His 90th Birthday.
Search for more papers by this authorXiangxiang Zhang
State Key Laboratory of Urban Water Resource and Environment, School of Chemistry and Chemical Engineering, Harbin Institute of Technology, 92 West Da-Zhi Street, Harbin, Heilongjiang, 150001 China
‡These authors contributed equally to this work.
†Dedicated to Professor Erkang Wang on the Occasion of His 90th Birthday.
Search for more papers by this authorWeichen Wang
State Key Laboratory of Urban Water Resource and Environment, School of Chemistry and Chemical Engineering, Harbin Institute of Technology, 92 West Da-Zhi Street, Harbin, Heilongjiang, 150001 China
Search for more papers by this authorMingdong Dong
Interdisciplinary Nanoscience Center (iNANO), Aarhus University, Aarhus, DK-8000 Denmark
Search for more papers by this authorCorresponding Author
Xiaojun Han
State Key Laboratory of Urban Water Resource and Environment, School of Chemistry and Chemical Engineering, Harbin Institute of Technology, 92 West Da-Zhi Street, Harbin, Heilongjiang, 150001 China
E-mail: [email protected]Search for more papers by this authorComprehensive Summary
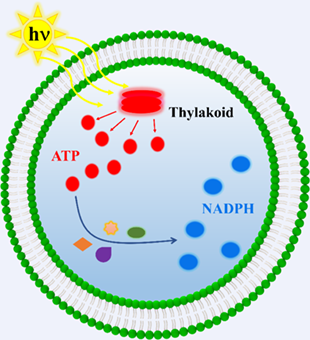
The bottom-up construction of self-powered artificial cells is significant to understand the energy supply and metabolism of nature cells. Here, we demonstrate an efficient manner to build thylakoid-containing artificial cells, which continuously convert light energy into chemical energy to supply adenosine 5’-triphosphate (ATP) under light illumination. The production of ATP supplies energy to promote the biological enzyme cascade reactions, where glucose is transformed into glucose 6-phosphate (G6P) under the catalysis of hexokinase (HK). G6P was further converted to gluconolactone 6-phosphate (PG) in the presence of 6-phosphate dehydrogenase (G6PDH), meanwhile NADP+ was converted to nicotinamide adenine dinucleotide phosphate (NADPH). The self-powered artificial cells were demonstrated to generate ATP and NADPH successively, which provided a way for building more complicated artificial cells.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202200482-sup-0001-Supinfo.pdfPDF document, 547.1 KB |
Appendix S1 Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Ryo, M.; Norikazu, I. Sustainable replication and coevolution of cooperative RNAs in an artificial cell-like system. Nat. Ecol. Evol. 2018, 2, 1654–1660.
- 2(a) Otrin, L.; Kleineberg, C.; Caire da Silva, L.; Landfester, K.; Ivanov, I.; Wang, M.; Bednarz, C.; Sundmacher, K.; Vidaković-Koch, T. Adv. Biosys. 2019, 3, 1800323; (b) Jeong, S.; Nguyen, H. T.; Kim, C. H.; Ly, M. N.; Shin, K. Toward Artificial Cells: Novel Advances in Energy Conversion and Cellular Motility. Adv. Funct. Mater. 2020, 30, 1907182.
- 3 Shin, K. Artificial cells containing sustainable energy conversion engines. Emerg. Top Life Sci. 2019, 3, 573–578.
- 4(a) Xu, Y. Q.; Fei, J. B.; Li, G. l.; Yuan, T. T.; Xu, X.; Wang, C. l.; Li, J. B. Optically Matched Semiconductor Quantum Dots Improve Photophosphorylation Performed by Chloroplasts. Angew. Chem. Int. Ed. 2018, 57, 6532; (b) Xu, Y. Q.; Fei, J. B.; Li, G. l.; Yuan, T. T.; Li, Y.; Wang, C. l.; Li, X. B.; Li, J. B. Enhanced Photophosphorylation of a Chloroplast-Entrapping Long-Lived Photoacid. Angew. Chem. Int. Ed. 2017, 56, 12903; (c) Wendell, D.; Todd, J.; Montemagno, C. Artificial photosynthesis in ranaspumin-2 based foam. Nano Lett. 2010, 10, 3231–3236; (d) Feng, X. Y.; Jia, Y.; Cai, P.; Fei, J. B.; Li, J. B. Coassembly of Photosystem II and ATPase as Artificial Chloroplast for Light-Driven ATP Synthesis. ACS Nano 2016, 10, 556–561; (e) Luo, T. J.; Soong, R.; Lan, E.; Dunn, B.; Montemagno, C. Photo-induced proton gradients and ATP biosynthesis produced by vesicles encapsulated in a silica matrix. Nat. Mater. 2005, 4, 220–224; (f) Lee, K. Y.; Park, S.; Lee, K. A.; Kim, S.; Kim, H.; Meroz, Y.; Mahadevan, L.; Jung, K.; Ahn, T. K.; Parker, K. K.; Shin, K. Photosynthetic artificial organelles sustain and control ATP-dependent reactions in a protocellular system. Nat. Biotechnol. 2018, 36, 530–535.
- 5 Li, F.; Wei, X. L.; Zhang, L.; Liu, C.; You, C.; Zhu, Z. G. Installing a Green Engine to Drive an Enzyme Cascade: A Light-Powered In Vitro Biosystem for Poly(3-hydroxybutyrate) Synthesis. Angew. Chem. Int. Ed. 2022, 61, e202111054.
- 6 Li, C.; Zhang, X. X.; Yang, B. Y.; Wei, F.; Ren, Y. S.; Mu, W.; Han, X. J. Reversible Deformation of Artificial Cell Colonies Triggered by Actin Polymerization for Muscle Behavior Mimicry. Adv. Mater. 2022, 2204039.
- 7 Adler, L.; Chen, C. H.; Koutalos, Y. Mitochondria contribute to NADPH generation in mouse rod photoreceptors. J. Biol. Chem. 2014, 289, 1519–1528.
- 8 Xu, J. Z.; Yang, H. K.; Zhang, W. G. NADPH metabolism: a survey of its theoretical characteristics and manipulation strategies in amino acid biosynthesis. Crit. Rev. Biotechnol. 2018, 1–16.
- 9(a) Stanton, R. C. Glucose-6-phosphate dehydrogenase, NADPH, and cell survival. IUBMB Life 2012, 64, 362–369; (b) Lunt, S. Y.; Vander Heiden, M. G. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu. Rev. Cell Dev. Biol. 2011, 27, 441–464.
- 10(a) Miller, T. E.; Beneyton, T.; Schwander, T.; Diehl, C.; Girault, M.; McLean, R.; Chotel, T.; Claus, P.; Cortina, N. S.; Baret, J.; Erb, T. J. Light-powered CO2 fixation in a chloroplast mimic with natural and synthetic parts. Science 2020, 368, 649–654; (b) Yang, B. Y.; Li, S. B.; Mu, W.; Wang, Z.; Han, X. J. Light-harvesting artificial cells containing cyanobacteria for CO2 fixation and further metabolism mimicking. Small 2022, 2201305.
- 11(a) Seigneurin-Berny, D.; Salvi, D.; Joyard, J.; Rolland, N. Purification of intact chloroplasts from Arabidopsis and spinach leaves by isopycnic centrifugation. Curr. Protoc. Cell Biol. 2008, 40, 3–30;
10.1002/0471143030.cb0330s40 Google Scholar(b) Strand, D. D.; Fisher, N.; Kramer, D. M. The higher plant plastid NAD(P)H dehydrogenase-like complex (NDH) is a high efficiency proton pump that increases ATP production by cyclic electron flow. J. Biol. Chem. 2017, 292, 11850–11860.
- 12 Zong, W.; Zhang, X. N.; Li, C.; Han, X. J. Thylakoid Containing Artificial Cells for the Inhibition Investigation of Light-Driven Electron Transfer during Photosynthesis. ACS Synth. Biol. 2018, 7, 945–951.
- 13(a) Fukuzumi, S.; Lee, Y.; Nam, W. Artificial Photosynthesis for Production of ATP, NAD(P)H, and Hydrogen Peroxide. ChemPhotoChem 2018, 2, 121; (b) Mechela, A.; Schwenkert, S.; Soll, J. A brief history of thylakoid biogenesis. Open Biol. 2019, 9, 180237; (c) Bernhard Teicher, H.; Vibe Scheller, H. The NAD(P)H dehydrogenase in barley thylakoids is photoactivatable and uses NADPH as well as NADH. Plant Physiol. 1998, 117, 525–532.
- 14 Paulsen, H. Chlorophyll a/b-Binding Proteins. Photochem. Photobiol. 1995, 62, 367–382.
- 15(a) Zhao, J. J.; Zhang, Y.; Zhang, X. X.; Li, C.; Du, H.; Sønderskov, S. M.; Mu, W.; Dong, M. D.; Han, X. J. Mimicking Cellular Metabolism in Artificial Cells: Universal Molecule Transport across the Membrane through Vesicle Fusion. Anal. Chem. 2022, 94, 3811–3818; (b) Fujii, S.; Matsuura, T.; Sunami, T.; Nishikawa, T.; Kazuta, Y.; Yomo, T. Liposome display for in vitro selection and evolution of membrane proteins. Nat. Protoc. 2014, 9, 1578–1591.
- 16 Chandra, A.; Prasad, S.; Iuele, H.; Colella, F.; Rizzo, R.; D'Amone, E.; Gigli, G.; del Mercato, L. L. Highly Sensitive Fluorescent pH Microsensors Based on the Ratiometric Dye Pyranine Immobilized on Silica Microparticles. Chem. - Eur. J. 2021, 27, 13279.
- 17 Arnon, D. I. Copper Enzymes in Isolated Chloroplasts. Polyphenoloxidase in Beta Vulgaris. Plant Physiol. 1949, 24, 1–15.




