Lewis Acid Enables Ketone Phosphorylation: Synthesis of Alkenyl Phosphonates
Corresponding Author
Xiao-Hong Wei
Key Laboratory for Utility of Environment-Friendly Composite Materials and Biomass in University of Gansu Province, College of Chemical Engineering, Northwest Minzu University, No. 1, Northwest Xincun, Lanzhou, Gansu, 730030 China
E-mail: [email protected]; [email protected]Search for more papers by this authorChun-Yuan Bai
Key Laboratory for Utility of Environment-Friendly Composite Materials and Biomass in University of Gansu Province, College of Chemical Engineering, Northwest Minzu University, No. 1, Northwest Xincun, Lanzhou, Gansu, 730030 China
Search for more papers by this authorLian-Biao Zhao
Key Laboratory for Utility of Environment-Friendly Composite Materials and Biomass in University of Gansu Province, College of Chemical Engineering, Northwest Minzu University, No. 1, Northwest Xincun, Lanzhou, Gansu, 730030 China
Search for more papers by this authorPing Zhang
Key Laboratory for Utility of Environment-Friendly Composite Materials and Biomass in University of Gansu Province, College of Chemical Engineering, Northwest Minzu University, No. 1, Northwest Xincun, Lanzhou, Gansu, 730030 China
Search for more papers by this authorZhen-Hua Li
Key Laboratory for Utility of Environment-Friendly Composite Materials and Biomass in University of Gansu Province, College of Chemical Engineering, Northwest Minzu University, No. 1, Northwest Xincun, Lanzhou, Gansu, 730030 China
Search for more papers by this authorYan-Bin Wang
Key Laboratory for Utility of Environment-Friendly Composite Materials and Biomass in University of Gansu Province, College of Chemical Engineering, Northwest Minzu University, No. 1, Northwest Xincun, Lanzhou, Gansu, 730030 China
Search for more papers by this authorCorresponding Author
Qiong Su
Key Laboratory for Utility of Environment-Friendly Composite Materials and Biomass in University of Gansu Province, College of Chemical Engineering, Northwest Minzu University, No. 1, Northwest Xincun, Lanzhou, Gansu, 730030 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Xiao-Hong Wei
Key Laboratory for Utility of Environment-Friendly Composite Materials and Biomass in University of Gansu Province, College of Chemical Engineering, Northwest Minzu University, No. 1, Northwest Xincun, Lanzhou, Gansu, 730030 China
E-mail: [email protected]; [email protected]Search for more papers by this authorChun-Yuan Bai
Key Laboratory for Utility of Environment-Friendly Composite Materials and Biomass in University of Gansu Province, College of Chemical Engineering, Northwest Minzu University, No. 1, Northwest Xincun, Lanzhou, Gansu, 730030 China
Search for more papers by this authorLian-Biao Zhao
Key Laboratory for Utility of Environment-Friendly Composite Materials and Biomass in University of Gansu Province, College of Chemical Engineering, Northwest Minzu University, No. 1, Northwest Xincun, Lanzhou, Gansu, 730030 China
Search for more papers by this authorPing Zhang
Key Laboratory for Utility of Environment-Friendly Composite Materials and Biomass in University of Gansu Province, College of Chemical Engineering, Northwest Minzu University, No. 1, Northwest Xincun, Lanzhou, Gansu, 730030 China
Search for more papers by this authorZhen-Hua Li
Key Laboratory for Utility of Environment-Friendly Composite Materials and Biomass in University of Gansu Province, College of Chemical Engineering, Northwest Minzu University, No. 1, Northwest Xincun, Lanzhou, Gansu, 730030 China
Search for more papers by this authorYan-Bin Wang
Key Laboratory for Utility of Environment-Friendly Composite Materials and Biomass in University of Gansu Province, College of Chemical Engineering, Northwest Minzu University, No. 1, Northwest Xincun, Lanzhou, Gansu, 730030 China
Search for more papers by this authorCorresponding Author
Qiong Su
Key Laboratory for Utility of Environment-Friendly Composite Materials and Biomass in University of Gansu Province, College of Chemical Engineering, Northwest Minzu University, No. 1, Northwest Xincun, Lanzhou, Gansu, 730030 China
E-mail: [email protected]; [email protected]Search for more papers by this authorMain observation and conclusion
An efficient Lewis acid enabled ketones phosphonylation to synthesize vinylphosphonates has been developed. This method relays on ketone hydrophosphonylation/α-hydroxy phosphonates unimolecular elimination (E1) dehydration cascade reaction sequence. Various C—P bond formation products were obtained in moderate to excellent yields with water as the only byproduct in the reaction.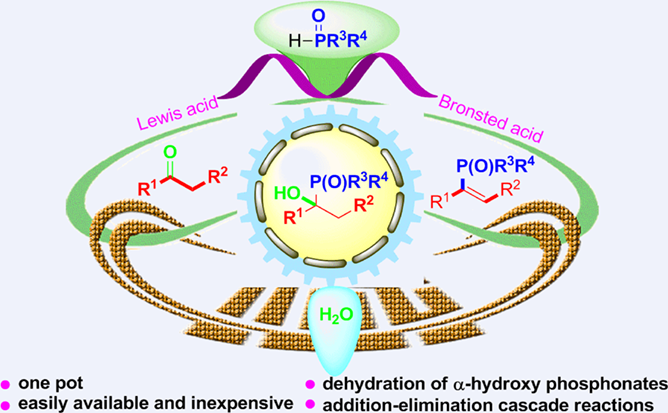
Supporting Information
| Filename | Description |
|---|---|
| cjoc202100083-sup-0001-Supinfo.pdfPDF document, 11.9 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Baumgartner, T.; Réau, R. Organophosphorus π-Conjugated Materials. Chem. Rev. 2006, 106, 4681–4727; (b) Duke, S. O.; Powles, S. B. Glyphosate: a once-in-a-century herbicide. Pest Manag. Sci. 2008, 64, 319–325; (c) Horsman, G. P.; Zechel, D. L. Phosphonate Biochemistry. Chem. Rev. 2017, 117, 5704–5783; (d) Martin, R.; Buchwald, S. L. Palladium-Catalyzed Suzuki-Miyaura Cross-Coupling Reactions Employing Dialkylbiaryl Phosphine Ligands. Acc. Chem. Res. 2008, 41, 1461–1473; (e) Mucha, A.; Kafarski, P.; Berlicki, Ł. Remarkable Potential of the α-Aminophosphonate/Phosphinate Structural Motif in Medicinal Chemistry. J. Med. Chem. 2011, 54, 5955–5980; (f) Nowack, B. Environmental chemistry of phosphonates. Water Res. 2003, 37, 2533–2546; (g) Peng, H.-Q.; Wang, Y.-Z. Effects of Boric Acid on Flame Retardancy of Intumescent Flame-Retardant Polypropylene Systems Containing a Caged Bicyclic Phosphate. In Fire and Polymers V, American Chemical Society, 2009, Vol. 1013, pp. 225–248; (h) Queffélec, C.; Petit, M.; Janvier, P.; Knight, D. A.; Bujoli, B. Surface Modification Using Phosphonic Acids and Esters. Chem. Rev. 2012, 112, 3777–3807; (i) Zbigniew, H. K.; Marcin, H. K.; Jozef, D.; Chris, V. S. Aminophosphonic Acids-Phosphorus Analogues of Natural Amino Acids. Part 1: Syntheses of α-Aminophosphonic Acids. Curr. Org. Chem. 2011, 15, 2015–2071; (j) Zhan, J.; Song, L.; Hu, Y. Combustion and Thermal Properties of Polylactide with an Effective Phosphate- Containing Flame-Retardant Oligomer. In Fire and Polymers V, American Chemical Society, 2009, Vol. 1013, pp. 205–223.
- 2(a) Ávila, D. S.; Gubert, P.; Palma, A.; Colle, D.; Alves, D.; Nogueira, C. W.; Rocha, J. B. T.; Soares, F. A. A. An organotellurium compound with antioxidant activity against excitotoxic agents without neurotoxic effects in brain of rats. Brain Res. Bull. 2008, 76, 114–123; (b) Motoyoshiya, J.; Ikeda, T.; Tsuboi, S.; Kusaura, T. Takeuchi, Y.; Hayashi, S.; Yoshioka, S.; Takaguchi, Y.; Aoyama, H. Chemiluminescence in Autoxidation of Phosphonate Carbanions. Phospha-1,2-dioxetanes as the Most Likely High-Energy Intermediates. J. Org. Chem. 2003, 68, 5950–5955; (c) Parmar, R.; Willoughby, J. L. S.; Liu, J.; Foster, D. J.; Brigham, B.; Theile, C. S.; Charisse, K.; Akinc, A.; Guidry, E.; Pei, Y.; Strapps, W.; Cancilla, M.; Stanton, M. G.; Rajeev, K. G.; Sepp-Lorenzino, L.; Manoharan, M.; Meyers, R.; Maier, M. A.; Jadhav, V. 5′-(E)-Vinylphosphonate: A Stable Phosphate Mimic Can Improve the RNAi Activity of siRNA-GalNAc Conjugates. ChemBioChem 2016, 17, 985–989; (d) Renata, G.; Marcin, S. Phosphonic Esters and their Application of Protease Control. Curr. Pharm. Design 2013, 19, 1154–1178; (e) Schwender, C. F.; Beers, S. A.; Malloy, E. A.; Cinicola, J. J.; Wustrow, D. J.; Demarest, K. D.; Jordan, J. Benzylphosphonic acid inhibitors of human prostatic acid phosphatase. Bioorg. Med. Chem. Lett. 1996, 6, 311–314; (f) Shi, Z.-D.; Yang, B.-H.; Zhao, J.-J.; Wu, Y.-L.; Ji, Y.-Y.; Yeh, M. Enantioselective hydrolysis of naproxen ethyl ester catalyzed by monoclonal antibodies. Bioorg. Med. Chem. 2002, 10, 2171–2175; (g) Valentine, W. J.; Kiss, G. N.; Liu, J.; E, S.; Gotoh, M.; Murakami-Murofushi, K.; Pham, T. C.; Baker, D. L.; Parrill, A. L.; Lu, X.; Sun, C.; Bittman, R.; Pyne, N. J.; Tigyi, G. (S)-FTY720-Vinylphosphonate, an analogue of the immunosuppressive agent FTY720, is a pan-antagonist of sphingosine 1-phosphate GPCR signaling and inhibits autotaxin activity. Cell. Signal. 2010, 22, 1543–1553; (h) Virieux, D.; Sevrain, N.; Ayad, T.; Pirat, J.-L. Chapter Two-Helical Phosphorus Derivatives: Synthesis and Applications. In Adv. Heterocycl. Chem., Eds.: Scriven, E. F. V.; Ramsden, C. A., Elsevier Academic Press, 2015, San Diego, Vol. 116, pp. 37–83; (i) Younes, S.; Baziard-Mouysset, G.; de Saqui-Sannes, G.; Stigliani, J. L.; Payard, M.; Bonnafous, R.; Tisne-Versailles, J. Synthesis and pharmacological study of new calcium antagonists, analogues of cinnarizine and flunarizine. Eur. J. Med. Chem. 1993, 28, 943–948.
- 3(a) Feng, J.-J.; Chen, X.-F.; Shi, M.; Duan, W.-L. Palladium-Catalyzed Asymmetric Addition of Diarylphosphines to Enones toward the Synthesis of Chiral Phosphines. J. Am. Chem. Soc. 2010, 132, 5562–5563; (b) Horner, L.; Hoffmann, H.; Wippel, H. G. Phosphororganische Verbindungen, XII. Phosphinoxyde als Olefinierungsreagenzien. Chem. Ber. 1958, 91, 61–63; (c) Juan, A. B.; Liliana, R. O. Recent Progress in the Horner-Wadsworth-Emmons Reaction. Curr. Org. Chem. 2015, 19, 744–775; (d) Ma, Y.-N.; Cheng, M.-X.; Yang, S.-D. Diastereoselective Radical Oxidative C–H Aminations toward Chiral Atropoisomeric (P, N) Ligand Precursors. Org. Lett. 2017, 19, 600–603; (e) Ma, Y.-N.; Li, S.-X.; Yang, S.-D. New Approaches for Biaryl-Based Phosphine Ligand Synthesis via P=O Directed C–H Functionalizations. Acc. Chem. Res. 2017, 50, 1480–1492; (f) Tang, W.; Zhang, X. New Chiral Phosphorus Ligands for Enantioselective Hydrogenation. Chem. Rev. 2003, 103, 3029–3070; (g) Wang, H.-L.; Hu, R.-B.; Zhang, H.; Zhou, A.-X.; Yang, S.-D. Pd(II)-Catalyzed Ph2(O)P-Directed C–H Olefination toward Phosphine–Alkene Ligands. Org. Lett. 2013, 15, 5302–5305.
- 4(a) Cheruku, P.; Paptchikhine, A.; Church, T. L.; Andersson, P. G. Iridium-N,P-Ligand-Catalyzed Enantioselective Hydrogenation of Diphenylvinylphosphine Oxides and Vinylphosphonates. J. Am. Chem. Soc. 2009, 131, 8285–8289; (b) Dong, K.; Wang, Z.; Ding, K. Rh(I)-Catalyzed Enantioselective Hydrogenation of α-Substituted Ethenylphosphonic Acids. J. Am. Chem. Soc. 2012, 134, 12474–12477; (c) He, S.-J.; Wang, J.-W.; Li, Y.; Xu, Z.-Y.; Wang, X.-X.; Lu, X.; Fu, Y. Nickel-Catalyzed Enantioconvergent Reductive Hydroalkylation of Olefins with α-Heteroatom Phosphorus or Sulfur Alkyl Electrophiles. J. Am. Chem. Soc. 2020, 142, 214–221.
- 5(a) Bou Orm, N.; Dkhissi, Y.; Daniele, S.; Djakovitch, L. Synthesis of 2-(arylamino)ethyl phosphonic acids via the aza-Michael addition on diethyl vinylphosphonate. Tetrahedron 2013, 69, 115–121; (b) Huang, H.; Zhu, H.; Kang, J. Y. Regio- and Stereoselective Hydrophosphorylation of Ynamides for the Synthesis of β-Aminovinylphosphine Oxides. Org. Lett. 2018, 20, 2778–2781; (c) Lefevre, N.; Brayer, J.-L.; Folléas, B.; Darses, S. Chiral α-Amino Phosphonates via Rhodium-Catalyzed Asymmetric 1,4-Addition Reactions. Org. Lett. 2013, 15, 4274–4276; (d) Ruiz, M.; Fernández, M. C.; Díaz, A.; Quintela, J. M.; Ojea, V. Diastereoselective Synthesis of 2-Amino-4-phosphono-butanoic Acids by Conjugate Addition of Lithiated Schöllkopf's Bislactim Ethers to Vinylphosphonates. J. Org. Chem. 2003, 68, 7634–7645; (e) Soller, B. S.; Salzinger, S.; Rieger, B. Rare Earth Metal-Mediated Precision Polymerization of Vinylphosphonates and Conjugated Nitrogen- Containing Vinyl Monomers. Chem. Rev. 2016, 116, 1993–2022.
- 6(a) Baumann, A. L.; Schwagerus, S.; Broi, K.; Kemnitz-Hassanin, K.; Stieger, C. E.; Trieloff, N.; Schmieder, P.; Hackenberger, C. P. R. Chemically Induced Vinylphosphonothiolate Electrophiles for Thiol–Thiol Bioconjugations. J. Am. Chem. Soc. 2020, 142, 9544–9552; (b) Buquoi, J. Q.; Lear, J. M.; Gu, X.; Nagib, D. A. Heteroarene Phosphinylalkylation via a Catalytic, Polarity-Reversing Radical Cascade. ACS Catal. 2019, 9, 5330–5335; (c) Chen, F.; Xia, Y.; Lin, R.; Gao, Y.; Xu, P.; Zhao, Y. Copper-Catalyzed Direct Twofold C–P Cross-Coupling of Unprotected Propargylic 1,4-Diols: Access to 2,3-Bis(diarylphosphynyl)-1,3-butadienes. Org. Lett. 2019, 21, 579–583; (d) Chen, T.; Zhao, C.-Q.; Han, L.-B. Hydrophosphorylation of Alkynes Catalyzed by Palladium: Generality and Mechanism. J. Am. Chem. Soc. 2018, 140, 3139–3155; (e) Gao, Y.; Wang, G.; Chen, L.; Xu, P.; Zhao, Y.; Zhou, Y.; Han, L.-B. Copper-Catalyzed Aerobic Oxidative Coupling of Terminal Alkynes with H-Phosphonates Leading to Alkynylphosphonates. J. Am. Chem. Soc. 2009, 131, 7956–7957; (f) Khemchyan, L. L.; Ivanova, J. V.; Zalesskiy, S. S.; Ananikov, V. P.; Beletskaya, I. P.; Starikova, Z. A. Unprecedented Control of Selectivity in Nickel-Catalyzed Hydrophosphorylation of Alkynes: Efficient Route to Mono- and Bisphosphonates. Adv. Synth. Catal. 2014, 356, 771–780; (g) Li, Y.-M.; Sun, M.; Wang, H.-L.; Tian, Q.-P.; Yang, S.-D. Direct Annulations toward Phosphorylated Oxindoles: Silver-Catalyzed Carbon-Phosphorus Functionalization of Alkenes. Angew. Chem. Int. Ed. 2013, 52, 3972–3976; (h) Ma, Y.-N.; Zhang, H.-Y.; Yang, S.-D. Pd(II)-Catalyzed P(O)R1R2-Directed Asymmetric C–H Activation and Dynamic Kinetic Resolution for the Synthesis of Chiral Biaryl Phosphates. Org. Lett. 2015, 17, 2034–2037; (i) Ren, L.; Ran, M.; He, J.; Xiang, D.; Chen, F.; Liu, P.; He, C.; Yao, Q. A Palladium-Catalyzed Decarboxylative Heck-Type Reaction of Disubstituted Vinylphosphonates in the Stereoselective Synthesis of Trisubstituted Vinylphosphonates. Eur. J. Org. Chem. 2019, 2019, 5656–5661; (j) Ren, W.; Zuo, Q.-M.; Niu, Y.-N.; Yang, S.-D. Palladium–NHC-Catalyzed Allylic Alkylation of Pronucleophiles with Alkynes. Org. Lett. 2019, 21, 7956–7960; (k) Wang, L.; Yang, Z.; Zhu, H.; Liu, H.; Lv, S.; Xu, Y. TEMPO and Silver-Mediated Intermolecular Phosphonylation of Alkenes: Stereoselective Synthesis of (E)-Alkenylphosphonates. Eur. J. Org. Chem. 2019, 2019, 2138–2142; (l) Yang, Q.; Li, C.; Cheng, M.-X.; Yang, S.-D. Palladium-Catalyzed Migratory Insertion of Isocyanides for Synthesis of C-Phosphonoketenimines. ACS Catal. 2016, 6, 4715–4719; (m) Yang, Q.; Yang, S.-D. Highly Efficient and Divergent Construction of Chiral γ-Phosphono-α-Amino Acids via Palladium-Catalyzed Alkylation of Unactivated C(sp3)–H Bonds. ACS Catal. 2017, 7, 5220–5224; (n) Yao, Q.; Ren, L.; Xiang, D.; Li, K.; Yan, B.; Ran, M.; He, J.; Zhao, L. The Photoinduced Metal-Free Hydrotrifluoromethylation of Vinyl Phosphonates or Phosphine Oxides. Eur. J. Org. Chem. 2019, 2019, 7475–7482.
- 7 Pergament, I.; Srebnik, M. Hydroboration of Unsaturated Phosphonic Esters: Synthesis of Boronophosphonates and Trisubstituted Vinyl- phosphonates. Org. Lett. 2001, 3, 217–219.
- 8(a) Schwan, A. L. Palladium catalyzed cross-coupling reactions for phosphorus–carbon bond formation. Chem. Soc. Rev. 2004, 33, 218–224; (b) Toshikazu, H.; Toshio, M.; Naoto, Y.; Yoshiki, O.; Toshio, A. Palladium-catalyzed New Carbon-Phosphorus Bond Formation. Bull. Chem. Soc. Jpn. 1982, 55, 909–913.
- 9(a) Fang, Y.; Zhang, L.; Jin, X.; Li, J.; Yuan, M.; Li, R.; Wang, T.; Wang, T.; Hu, H.; Gu, J. α-Phosphonovinyl Arylsulfonates: An Attractive Partner for the Synthesis of α-Substituted Vinylphosphonates through Palladium-Catalyzed Suzuki Reactions. Eur. J. Org. Chem. 2016, 2016, 1577–1587; (b) Fang, Y.; Zhang, L.; Li, J.; Jin, X.; Yuan, M.; Li, R.; Wu, R.; Fang, J. Applications of α-Phosphonovinyl Tosylates in the Synthesis of α-Arylethenylphosphonates via Suzuki-Miyaura Cross-Coupling Reactions. Org. Lett. 2015, 17, 798–801.
- 10(a) Jena, N.; Kazmaier, U. Synthesis of Stannylated Allyl- and Vinylphosphonates via Molybdenum-Catalyzed Hydrostannations. Eur. J. Org. Chem. 2008, 2008, 3852–3858; (b) Konno, T.; Kinugawa, R.; Morigaki, A.; Ishihara, T. An Efficient Protocol for the Stereoselective Construction of Multisubstituted Fluorine-Containing Alkenes. A Palladium-Catalyzed Bisstannylation of Fluorinated Internal Alkynes. J. Org. Chem. 2009, 74, 8456–8459.
- 11 Chen, H.-X.; Huang, L.-J.; Liu, J.-B.; Weng, J.; Lu, G. Synthesis of Terminal Vinylphosphonates via Dbu-Promoted Tandem Phospha-Michael/Elimination Reactions. Phosphorus Sulfur 2014, 189, 1858–1866.
- 12 Huang, T.; Saga, Y.; Guo, H.; Yoshimura, A.; Ogawa, A.; Han, L.-B. Radical Hydrophosphorylation of Alkynes with R2P(O)H Generating Alkenylphosphine Oxides: Scope and Limitations. J. Org. Chem. 2018, 83, 8743–8749.
- 13 Zhou, X.; Liu, Y.; Chang, L.; Zhao, J.; Shang, D.; Liu, X.; Lin, L.; Feng, X. Highly Efficient Synthesis of Quaternary α-Hydroxy Phosphonates via Lewis Acid-Catalyzed Hydrophosphonylation of Ketones. Adv. Synth. Catal. 2009, 351, 2567–2572.
- 14 Unoh, Y.; Hirano, K.; Miura, M. Metal-Free Electrophilic Phosphination/Cyclization of Alkynes. J. Am. Chem. Soc. 2017, 139, 6106–6109.
- 15 Guo, T.; Zhang, L.; Fang, Y.; Jin, X.; Li, Y.; Li, R.; Li, X.; Cen, W.; Liu, X.; Tian, Z. Visible-Light-Promoted Decarboxylative Giese Reactions of α-Aryl Ethenylphosphonates and the Application in the Synthesis of Fosmidomycin Analogue. Adv. Synth. Catal. 2018, 360, 1352–1357.
- 16(a) Anitha, M.; Kotikalapudi, R.; Swamy, K. C. K. FeCl3 catalysed regioselective allylation of phenolic substrates with (α-hydroxy)allylphosphonates. J. Chem. Sci. 2015, 127, 1465–1475; (b) Nilsson, J.; Kraszewski, A.; Stawinski, J. Reinvestigation of the 31P NMR evidence for the formation of diorganyl phosphoropyridinium intermediates. J. Chem. Soc.-Perkin Trans. 2 2001, 2263–2266; (c) Dabkowski, W.; Michalski, J.; Skrzypczynski, Z. Anhydrides of phosphorus and sulfur acids, 2. Mixed anhydrides of phosphoric, phosphonic, and phosphinic acids with sulfonic acids and sulfuric Monoimidazolide. New methods of synthesis, novel structures, phosphorylating properties. Chem. Ber. 1985, 118, 1809–1824; (d) Pallikonda, G.; Chakravarty, M. Triflic acid mediated functionalization of α-hydroxyphosphonates: route for sulfonamide phosphonates. RSC Adv. 2013, 3, 20503–20511; (e) Pan, J.; Zhao, R.; Guo, J.; Ma, D.; Xia, Y.; Gao, Y.; Xu, P.; Zhao, Y. Three-component 3-(phosphoryl)methyl-indole synthesis from indoles, H-phosphine oxides and carbonyl compounds under metal-free conditions. Green Chem. 2019, 21, 792–797.




