How to Decarbonize Our Energy Systems: Process-Informed Design of New Materials for Carbon Capture
Abstract
Decarbonisation from a variety of industrial and power emission sectors highlights a marked need for capture technologies that can be optimized for different CO2 sources and integrated into an equally diverse range of applications of captured CO2 as a feedstock. Some capture technologies are already operated at an industrial scale but may not be optimal for all required applications. Advanced tailored sorbent-based technologies allow flexible operation and reduced costs as they offer higher capture capacities and significantly lower energy penalties than the state-of-the-art systems. To accelerate the discovery, development, and deployment of novel advanced materials, it is critically important that efforts between experimentalists, theoreticians, and process engineers are coordinated. The PrISMa project addresses this challenge by integrating materials design with process design and environmental considerations to allow for tailor-making carbon capture solutions optimally tuned for local sources and sinks. In this article, we highlight some of the recent results obtained with the PrISMa platform.
1 Introduction
The need to reduce carbon emissions is one of the most pressing challenges of our generation. In particular, at present, we still rely on fossil energy for a large fraction of our energy demand. Transitioning to a carbon-free economy will require time. Carbon capture, utilization, and storage (CCUS) is, at present, the only technology that allows us to reduce CO2 emissions while transitioning to a carbon-free economy 1. In fact, in a net zero world, we have to capture CO2 from many different sources to ensure we close the carbon loop and, for example, use the CO2 captured from waste incineration as a source of carbon for the chemical industry. In addition, given the lack of progress in decreasing our carbon emissions, there is an increasing need for technologies that reduce the CO2 levels in the atmosphere 2, 3.
If we envision a world where we need to capture CO2 from all kinds of sources, we need to identify the optimal technology for a particular source. In addition, different applications or storage solutions of CO2 require a gas stream at different purities and conditions. The optimal capture technology will depend on the particular CO2 sink. At present, the default technology is amine-based capture. This technology was initially developed to remove CO2 from natural gas (gas sweetening) and has been further optimized for capturing CO2 from flue gasses. One of the critical challenges for amine-based carbon capture is that most flue gasses contain oxygen, and this oxygen reacts with the amines, causing the degradation of the amine solution. In addition, the binding of CO2 with amines is relatively strong; hence one needs significant amounts of energy to regenerate the amine solution. Considerable research is being carried out to optimize amines for flue gas capture.
An alternative for liquid absorption is solid adsorption. Sorbent-based capture technologies have the potential to significantly reduce the effective price of carbon, i.e., reduce the energy penalty (OPEX) and/or equipment costs (CAPEX) 4. In addition, the number of possible solid sorbents has, due to the broad space and diversity of novel nanoporous materials (e.g., metal-organic frameworks (MOFs), covalent organic frameworks (COFs)), grown exponentially over the past decade (Fig. 1). MOFs are synthesized by combining organic ligands with metal nodes. Chemists have synthesized over 90 000 MOFs 5. Of these structures, over 10 000 are porous crystals and could potentially be used in a carbon capture process. In addition, using computational techniques, researchers have been able to predict novel MOF structures in silico. With these so-called material genomics methods, Kim et al. have predicted over a trillion possible MOFs structures 6.
Having so many materials to choose from allows us to tailor-make materials for different carbon capture applications. But the main question that remains is: can we identify the optimal material for a given source and sink of CO2? The conventional trial and error approach of synthesizing and testing does not work for a trillion materials. A first step towards addressing this challenge has been taken by computational groups, who used molecular simulation techniques to predict the thermodynamic properties of gasses adsorbed in these materials 7. For example, Lin et al. 8 screened hypothetical zeolites and ZIF structures using the parasitic energy as a metric. Snurr and co-workers 7, 9, 10 carried out similar MOF screening studies. Keskin and co-workers 11-14 investigated the application of these MOFs in membranes for CO2 separations. Each screening study used brute-force molecular simulations to screen libraries of thousands of materials. These initial studies used different metrics to rank a material, such as the capacity to adsorb CO2 at different pressures 7, 9, and more recently, groups are using metrics that are related to an actual capture process 10, 15. As they all compute basis adsorption thermodynamic data for each material, we can use these data to rank materials using simple key performance indicators (KPIs). Such material KPIs are useful for eliminating poorly performing materials (low capacity, low selectivity, etc.). However, as they are ignorant of any details of the process in which these materials need to be used, one cannot directly use these screening studies to identify the best material; the optimal material is not independent of the source of CO2.
For example, to decarbonize different industrial sectors, the most efficient and cost-effective capture technology will depend on the characteristics of the CO2 source, the specific location, and local resources. If the concentration of CO2 is high, the design and operation of the capture plant will differ from those cases where the CO2 concentration on the gas stream is low, and hence the selection of the materials will be entirely different. Another example is if one would like to install a capture plant off-shore, where space is one of the most important criteria, and one would like to identify the cheapest option given the available space.
Making the link between materials properties and process design has become an active area of research lately. Subraveti et al. 16 investigated the cost limits of two single-stage pressure-vacuum swing adsorption (PVSA) cycles for post-combustion CO2 capture. In that study, Subraveti et al. assumed that the change of the material mainly impacts the adsorption isotherms, as described with a dual-site Langmuir (DSL) isotherm. From the optimization of the cost of the process, they determined the ideal parameters of the DSL model. This approach risks converging on a material that cannot be synthesized. To reduce this risk, the DSL parameters were bounded by values found in a set of different MOFs. Subraveti et al. concluded that the ‘‘ideal'' material should have a linear CO2 adsorption isotherm and N2 adsorption close to zero. An interesting next step of this work is identifying a material with such adsorption properties.
This next step requires a direct link of materials, process simulations, techno-economics, and life cycle analysis. Making this link is the vision of the PrISMa platform (Process-informed designed of tailor-made sorbent materials for energy-efficient carbon capture, www.act-ccs.eu/prisma, https://prisma.hw.ac.uk). PrISMa is an international effort supported by the ACT programme (Accelerating CCS Technologies, Horizon2020 Project No 294766). PrISMa is a collaboration of research teams from Heriot-Watt University (HWU) in the UK, École Polytechnique Fédérale de Lausanne (EPFL), as well as Eidgenössische Technische Hochschule Zurich (ETH) in Switzerland, and Lawrence Berkeley National Laboratory (LBNL) in the USA. PrISMa aims to develop the science needed to accelerate the transition of energy and industrial sectors to a low-carbon economy. To achieve that aim, PrISMa has developed a technology platform to tailor-make cost-efficient carbon capture solutions for various CO2 sources and CO2 use/destinations.
2 The PrISMa Materials Discovery Workflow
In the PrISMa platform, advanced techno-economic material screening models are used to identify and quantify the relevant process KPIs, e.g., CO2 purity, recovery, productivity, and energy consumption, that should lead to improved carbon capture processes. These performance indicators are further translated into targets for the design of novel materials. This allows us to deeply understand the material properties and requirements underpinning sorbent-based carbon capture processes.
The material properties are key variables that link the system and molecular levels and are targeted to meet the specific criteria set by the KPIs. Besides the cost and to enable efficient material selection, environmental aspects have to be considered within the design as early as possible. For this purpose, we investigate how KPIs can be derived to screen the environmental impacts of the materials' synthesis, use, and disposal phases and the corresponding processes. To assess the environmental impact of the use and disposal phase, we analyse the relationship between the environmental impacts and process-based KPIs. From the identified relationships, we derive environmental KPIs for the use and disposal phase. To synthesize sorbents, we explore two approaches: 1) a first-principles approach by representing the actual synthesis process using simplified process simulations, and 2) a data-driven approach where environmental impacts are predicted from molecular descriptors, e.g., using machine-learning algorithms. Similar approaches have been successful in predicting the environmental impact of solvents in the early design phase 17. From the analysis of the environmental impacts of the synthesis, use, and disposal phase of the materials, we derive environmentally optimal ranges of sorbent properties from serving as additional environmental KPIs for the materials screening. Once accurate relationships are identified to capture the environmental impact, the environmental KPIs are also considered for materials screening.
All the KPI targets obtained from the different aspects included in the platform, i.e., technological, economic, and environmental, are rooted in our conviction that significant progress can be made if there is direct feedback between fundamental research and real-life applications, which allows us to answer questions like: What are the properties we need to predict for a novel material, and what is the accuracy needed? Do we have a sufficiently fundamental understanding of the adsorption behaviour of all possible contaminants? What if we cannot design a material that will meet all criteria, but we obtain a set of materials that are close; what is the best engineering solution for these materials? Or what if we find a material that requires an entirely different design? How does it change process design if we can make materials on demand?
The interrogation of the vast materials and process design space that requires answering all those questions generates an enormous amount of performance data for thousands of materials tested in-silico in many different processes. As a result, we can make use of big-data science and develop multiscale workflows that enable us to find correlations between the properties of materials and their performance in a process. The size and complexity of the data require the development of machine-learning methods to enable its analysis and mining (Fig. 2). For instance, we directly investigate the integration of data-driven materials approaches into rigorous process modelling and optimization of the considered CO2 capture processes. The integration will enable an efficient assessment of materials on the process level because time-consuming molecular-scale calculations are avoided. We also explore if and how process-level feedback from machine learning can link novel process-based KPIs directly to molecular properties or even enable the direct optimization of materials on the process level. Combining goal programming approaches with data-driven analysis should enable the extraction of molecular features leading to optimal process performance and thereby guiding the materials development.

3 The Power of an Integrated Platform for Materials Discovery
We used an earlier version of the PrISMa platform to carry out a screening study to identify a material that can capture CO2 in wet flue gasses 18. In this study, we generated 300 000 materials in silico to identify those materials that perform better than zeolite 13X. In this early version, we used CO2 capacity and H2O/N2 selectivity as metrics. We subsequently used a data-science method to identify the “adsorbaphore,” the molecular characteristics of the CO2 adsorption site that the top-performing materials have in common. Interestingly, our platform predicted that one class of these adsorbaphores was insensitive to the presence of water. Subsequent synthesis of a metal-organic framework that incorporated this adsorbaphore confirmed this prediction. In addition, capture tests showed that this material outperformed the commercial materials.
One of the interesting side-effects of the PrISMa platform is that we screen a little over thousand materials 19. For these screenings, we used molecular simulations 20 to predict the pure component adsorption isotherms, and we used ideal adsorbed solution theory (IAST) to predict the mixture isotherms. These steps are similar to what is common practice in the field. In addition, it is well known that one has to be careful to correctly fit the pure component isotherms. One typically tests several models as some adsorption behaviour can only be described with some particular equations. However, with thousands of materials, it is impossible to decide by hand how to best fit the adsorption isotherms for each material. Therefore, we have developed a workflow that gives us the optimal IAST predictions without having to fit the data. This workflow is based on the PyIAST program developed by Simon et al. 21 and is described in detail by Moubarak et al. 22.
From a process simulation point of view, predicting mixture isotherms using IAST requires more CPU time compared to using an analytical expression, such as DSL isotherms. This can become problematic if one needs to optimize the process. The results reported by Moubarak et al. show that, in particular, at low concentrations of CO2, the DSL predictions cannot be trusted. Hence it is essential to address the CPU issue. For example, by fitting the IAST mixture predictions to an analytical expression or pre-compute the mixture isotherm on a grid and using interpolation techniques 23. An alternative approach is the use of data science methods to develop a surrogate model. Such models have already been successfully used for full physical models, where the CPU time for optimization is also a problem 24-26.
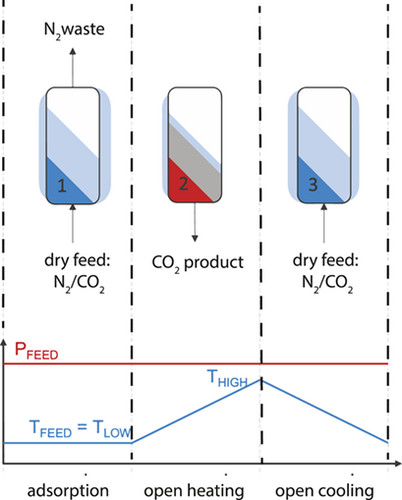
One of the main impacts of the PrISMa platform is that it sets a baseline for a suite of technologies that can find optimal solutions for all different sources of CO2, including medium and small local sources, which are highly desirable in a world that cannot afford to emit any additional carbon into the atmosphere. A secondary, but scientifically not less important, aim is that we can identify the importance of the different parameters. For this, we developed a simple three-step temperature swing adsorption (TSA) process to capture CO2 from a coal-fired power plant.
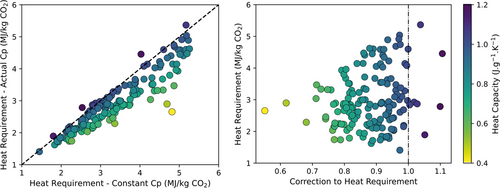
With the platform, we are able to evaluate the performance of any adsorbent material and rank the material for some typical process key performance indicators such as purity, productivity, energy requirements, etc. We are also able to study the sensitivity of the different parameters systematically. One of the parameters that is important for the energy requirement is the heat capacity (Cp). The higher the heat capacity, the more energy is required to heat the material in the open heating step (see Fig. 3). Unfortunately, the heat capacity of MOFs has been measured for only a few materials. Hence, in our PrISMa platform, we assumed a constant value for all MOFs. This motivated us to develop a machine-learning approach to predict the heat capacity of the different porous materials (MOFs, zeolites, covalent-organic frameworks) 19. Fig. 4 compares the outcomes of the PrISMa platform with a constant Cp and with the Cp obtained from the machine learning model of Moosavi et al. 19. Interestingly, for almost all materials, this gave a lower value of the energy requirements.
The heat capacity is an example of a property we lack data. For those properties for which we have experimental data, there is always some uncertainty in the data. Cleeton et al. 27 systematically investigate the effects of the uncertainty on the estimate of the performance of a material in a pressure swing adsorption (PSA) process. This uncertainty in the experimental data implies that one does not have, for example, a single adsorption isotherm but a distribution of isotherms. Cleeton et al. developed a workflow to quantify how this uncertainty propagates in the screening results for the PSA process. Instead of having a single Pareto front Cleeton et al. argue that one can capture this uncertainty by considering a Pareto cloud.
Our results so far confirm the importance of bridging materials discovery with process design for accelerating the deployment of advanced sorbent-based carbon capture technologies. The successful integration of all these concepts is key to ensuring the breakthroughs in separation technologies that are required in the coming decades to achieve our net zero greenhouse emission targets.
4 Conclusions
The ACT-funded PrISMa project provides a technology platform that allows us to evaluate the performance of a large number of potential sorbents for carbon capture applications. Modern chemistry has the potential to generate a nearly infinite number of materials, and it is impossible to evaluate all these materials by trial and error. The first step of this platform is an in silico screening of materials, which allows us to rank materials in terms of process key performance indicators. We plan to extend our platform to include techno-economics and life-cycle analysis.
To link materials, process, techno/economics, and life cycle analysis, an important step is to ensure that we can compute a consistent set of KPIs for a fixed process and process conditions. This will allow us to rank the materials for the different KPIs. However, in practice, one would like to optimize both the process and process parameters for each material. For such an optimization, brute-force optimization is too time-consuming, and we need to develop alternative techniques. This is an active area of research where one can expect significant progress in the coming years. For example, the approaches so far have explored the link between material properties and process parameters; in these studies, either machine learning methods 24 are used to avoid expensive optimization or superstructure-based optimization 28.
Through integrating materials design, process design, and environmental considerations, we will change the paradigm of how novel materials are developed for carbon capture applications. Additionally, this approach can be extended to other separation processes, so we expect PrISMa to have a long-term impact on the way we integrate fundamental science and process engineering.
Acknowledgements
The PrISMa Project (No 299659) is funded through the ACT programme (Accelerating CCS Technologies, Horizon2020 Project No 294766). Financial contributions made from: Department for Business, Energy & Industrial Strategy (BEIS) together with extra funding from NERC and EPSRC research councils, United Kingdom; The Research Council of Norway, (RCN), Norway; Swiss Federal Office of Energy (SFOE), Switzerland; and US-Department of Energy (US-DOE), USA, are gratefully acknowledged. Financial support from TOTAL and Equinor is also gratefully acknowledged. This work is also supported by the UKRI ISCF Industrial Challenge within the UK Industrial Decarbonisation Research and Innovation Centre (IDRIC) award number: EP/V027050/1.
Symbols used
-
- Cp [J kg−1K−1]
-
heat capacity
Abbreviations
-
- ACT
-
Accelerating CCS Technologies
-
- CCS
-
Carbon Capture and Storage
-
- CCUS
-
Carbon Capture, Utilization, and Storage
-
- CPU
-
Central Processing Unit
-
- COF
-
Covalent-Organic Frameworks
-
- DSL
-
Dual-Site Langmuir
-
- EPFL
-
École Polytechnique Fédérale de Lausanne
-
- ETH
-
Eidgenössische Technische Hochschule Zurich
-
- HWU
-
Heriot-Watt University
-
- IAST
-
Ideal Adsorbed Solution Theory
-
- KPI
-
Key Performance Indicator
-
- MOF
-
Metal-Organic Framework
-
- LBNL
-
Lawrence Berkeley National Laboratory
-
- PrISMa
-
Process-Informed design of tailor-made Sorbent Materials for energy-efficient carbon capture
-
- PSA
-
Pressure Swing Adsorption
-
- TSA
-
Temperature Swing Absorption




