Recovery of Al, Co, Cu, Fe, Mn, and Ni from spent LIBs after Li selective separation by COOL-Process – Part 2: Solvent Extraction from Sulphate Leaching Solution
Abstract
The market for lithium-ion batteries (LIB) is experiencing substantial growth, what is owed to electromobility and the growing demand for cordless devices. Securing the raw material base for LIB is mandatory to achieve the objectives of EU commission's Green Deal. This implies not only sufficient access to lithium but also the accompanying metals, above all Co, Ni, Mn. After preceding recovery of Li2CO3 by the COOL-Process and subsequent acid digestion of the Li-free black mass, value metals, such as Co, Cu, Ni, and Mn were recovered by counter-current solvent extraction. Fe (98.5 ± 0.65 %), Co/Mn (99.8 ± 0.78 %; 99.9 ± 1.11 %), Al (99.7 ± 1.07 %) and Cu/Ni (97.8 ± 1.46 %; 98.6 ± 1.32 %) were selectively extracted with high discriminatory power using cationic exchangers and solvating extractants such as D2EHPA and TBP, respectively. This way, a complete, holistic recycling process for LIB is at hand that allows for recovering housing material, lithium and the accompanying metals almost quantitatively.
1 Introduction
Efficient recycling of lithium-ion batteries (LIBs) is a key step towards achieving a sustainable and resource-efficient economy. In this sense, the supply of all necessary metals (especially Co, Cu, Li, Mn, and Ni) can be ensured, and material cycles can be closed, thus contributing to a circular economy. To the best of our knowledge, the LIB recycling processes have in common that lithium can be separated only at the end of the process chain. This is associated with high losses, as well as impurities in lithium carbonate (Li2CO3). For LIB recycling we propose a to step process: (1) recovery of Li by selective leaching with supercritical CO2 according to the COOL-Process 1. This was achieved. (2) Recovery of the accompanying metals contained in the solid residue, such as Co, Cu, Mn, and Ni. In general, hydrometallurgical processes involving solvent extraction (SX) stages are common strategies to recover them from secondary raw material streams. In the case of LIBs however, recovery of these metals typically confines to black mass, while some very few studies only have focused on holistic processes that allow for recovering and utilizing the entire battery 2, 3. The aim of our work therefore was to investigate whether a cost-efficient zero-waste is viable in LIB recycling.
The recovery of various valuable metals from sulfuric, choride or citric media has been described exhaustively in several publications, where mainly cationic exchangers and chelating extractants come into play. Innocenzi et al. recovered selectively Ni from NiMH batteries using 20 vol % bis(2-ethylhexyl)phosphoric acid (D2EHPA) diluted in n-dodecane 4 in sulfuric media. Ni was separated from a mixture of Ni/Zn/Mn after two cross-flow solvent extractions. D2EHPA was also used by Chen and Zhou who extracted Mn from spent LIBs in citric acid media 2. In this case, the acidic organic extractant was saponified by using NaOH. 98 % of Mn was extracted by 20 vol % Na-D2EHPA at pH 4 and stripped using 0.2 mol L−1 H2SO4. A chelating extractant called LIX63 was used in combination with D2EHPA to separate Ni and Co from chloride leaching solution of nickel laterite ore 5. 62 % of Ni and 96 % of Co were recovered by 3:7 D2EHPA/LIX63. 8 mol L−1 HCl solution was required to strip both metals from the loaded phase. Co can be separated by Co-Ni mixture using amines as extractant reagents. Joo et al. demonstrated that an antagonistic effect of Versatic 10 acid (neodecanoic acid) to PC88 A (2-ethylhexyl phosphoric acid-mono-2-ethylhexyl ester) allows a higher recover efficiency for Mn 6. 11.7 g L−1 of this metal was separated from a Co/Ni/Mn/Li mixture from spent LIBs after four-stages counter-current extraction process using 25 vol % Versatic 10 acid/20 vol % PC88A at pH 4.5, one scrubbing stage using EDTA, and two stripping stages with H2SO4. Bis(2,4,4-trimethylpentyl)phosphinic acid (Cyanex 272) is another well-known cationic exchanger (not as acidic as D2EHPA), which is able to separate Co from a Co-Ni mixture from spent NiMH batteries in sulfuric media at pH 4.5 7. The main advantage of using this organic extractant compared to D2EHPA or PC88A is its comparably low concentration in the stripping solution. Kang et al. recovered 28 g L−1 Co from spent LIBs by using 50 % saponified 0.4 mol L−1 Cyanex 272 at an equilibrium pH 6 by two-stages counter-current operation with aqueous/organic (A/O) ratio of 1:2 3.
There have been several efforts to develop processes for the recycling of spent LIBs. However, most of these processes only target at the higher value metals cobalt and nickel instead of lithium mainly due to the price differences (19,373 USD t−1 Ni; 52,500 USD t−1 Co; 13,720 USD t−1 Li2CO3 8). Consequently, at present there is no process operated on a large scale for the recycling of lithium. Umicore S.A. in fact runs the so far largest scale LIB recycling process in their Hoboken plant in Belgium. Yet, their pyrometallurgical VAL‘EAS process targets at recovering Co and Ni, while Li accumulates in the slag 9, 10. The recently launched EarLiMet-project aims at recovering Li from those slags 11. Almost the same applies to the TOXCO Inc. process (Anaheim, USA), which in a low temperature decomposition targets at Co and Ni, too 12. The Vacuum Thermal Recycling (VTR) process by Accurec Recycling GmbH in Krefeld (Germany) pursues a combination of mechanical treatment and pyrometallurgical as well as hydrometallurgical process steps. Target products are Ni, Co, Li2CO3, Fe, Al, Cu, thus aiming at the entire metal content. The plant has a capacity to handle 4000 tons per year, however, there is no information available, whether this rather complex process can be operated economically under the given price situation of 13,885 USD t−1 bg-Li2CO3 in June 2021 8, 13. A somewhat more future directed approach is the Lithorec project (Braunschweig University of Technology, Germany), which targets both at the entire metal content and the housing components (Al, steel). It has been developed to the pilot scale but is no longer pursued 14. To the best of our knowledge, the COOL process is the most advanced approach 1. It targets at the entire metal content as well as housing materials, too. As an origin-independent hybrid process however, it is capable of digesting not only black mass, but also lithium cathode materials from 1st generation lithium batteries as well as primary raw materials, such as spodumene, zinnwaldite or lepidolite. It can be well combined with other mechanical treatment process, such as the one developed by Duesenfeld GmbH (Wendeburg, Germany). However, although Li recovery has been successfully established for different types of LIB, recovery of the accompanying metals so far has been the Achilles heel.
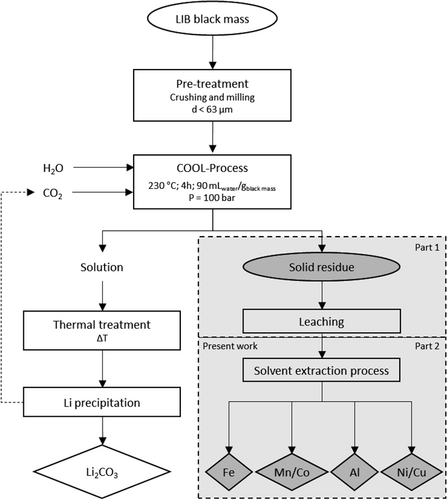
This study therefore aims at recovering all valuable metals contained in the solid residue (Li-free black mass obtained by COOL-Process stage) via solvent extraction processes (Fig. 1) after preceding digestion with sulfuric acid 15. As a result, a holistic approach allowing for selectively working up the leaching solutions for their metal content has been developed, what contributes to fully recycle LIBs.
2 Material and Methods
2.1 Leachate Composition and Reagents
Solvent extraction experiments were done with an aqueous phase the elemental composition of which is given in Tab. 1. The solid residue was treated with aqua regia in a liquid/solid ratio (L/S) of 100 at 180 ± 2 °C for 30 min using a Microwave MARS 6, CEM Corporation, London, UK. The metals concentration was determined by atomic emission spectrometry with inductively coupled plasma (ICP-OES, Optima 4300 DV, Perkin Elmer, MA, USA).
|
|
Concentration [mg/L] |
|---|---|
|
Al |
1.2 ± 0.02 |
|
Co |
3.7 ± 0.12 |
|
Cu |
3.1 ± 0.48 |
|
Fe |
0.8 ± 0.21 |
|
Mn |
38.3 ± 0.91 |
|
Ni |
14.4 ± 2.1 |
Preliminary tests were conducted using different kinds of organic extractants to find the most suitable one for the selective separation of Al/Co/Cu/Fe/Mn/Ni mixture. Commercial extractants such as D2EHPA (Sigma-Aldrich, MO, USA), tributyl phosphate (TBP, Merck KGaA, Darmstadt, Germany), Cyanex 923 (Cy923, a mixture of four trialkylphosphine oxides, Cytec Canada Industries Inc., NJ, USA), trioctylmethylammonium chloride (Aliquat 336, Ali336, Merck KGaA, Darmstadt, Germany), and 1,3-diphenyl-1,3-propandione (DPPD, Sigma-Aldrich, MO, USA) were the five extractants used in the screening experiment. Kerosene was used as a diluent (Sigma-Aldrich, MO, USA).
2.2 Solvent Extraction
10 mL of the aqueous phase (AP) were equilibrated with 10 mL of organic phase (OP) into a separatory funnel. The experiments were conducted using a horizontal mechanical shaker (Gesellschaft für Labortechnik mbH, model 3017, Burgwedel, Germany) at 180 rpm at room temperature (20 ± 2 °C) for 30 min to ensure that the equilibrium has been reached. The aqueous phase was removed and stored for further analyses once both phases were separated after the shaking. Afterwards, the equilibrium pH was measured by pH meter Hanna Instruments edge® blu HI2202-02 (RI, USA). The loaded phase was treated with a stripping solution, which depends on the extractant type and its extraction mechanisms. Water, HCl (37 %, VWR International, PA, USA) and H2SO4 (95 %, Thermo Fischer Scientific, MA, USA) were the stripping reagents used for re-extracting the metal 2, 5, 16-18. This stripping stage was performed using the same procedure that the extraction stage. Each experiment was carried out two times. Metal concentrations in the aqueous phases were determined by atomic emission spectrometry with inductively coupled plasma (ICP-OES, Optima 4300 DV, Perkin Elmer, MA, USA). If not stated otherwise, all experiments were done in duplicate.
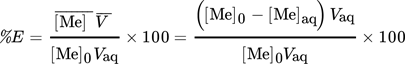 (1)
(1)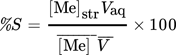 (2)
(2)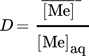 (3)
(3)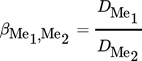 (4)
(4)where [Me]0 and [Me]aq refer to the initial and the equilibrium concentration of the metal in the aqueous phase. Vaq is the volume of the aqueous phase under initial, equilibrium, and stripping conditions, as no volume variation was observed in extraction and stripping steps. 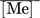 is the concentration of the metal in the loaded organic phase obtained by mass balance and
is the concentration of the metal in the loaded organic phase obtained by mass balance and  is the volume of the organic phase. [Me]str is the metal concentration of the stripping phase once the equilibrium is reached.
is the volume of the organic phase. [Me]str is the metal concentration of the stripping phase once the equilibrium is reached.
3 Results and Discussion
3.1 Solvent Extraction
Whether or not metal recovery from back mass is economically viable depends on the ratio of the intrinsic metal value over process costs. It was therefore a major breakthrough to keep leaching costs low in using sulfuric acid as digestion acid, although a compromise had to be made in terms of leaching efficiency 15. One may clearly find leaching conditions that are suitable to get a somewhat higher overall leaching efficiency, yet at far higher costs that most likely overcompensate for the metal value and thus render at least the black mass recycling stage a minus business.
For the extraction of the accompanying metals extraction experiments were carried out with five different organic extractants, which had proven successful in other recycling processes 19. The leachate had been obtained from acid digesting the Li-free solid residue resulting from the COOL-Process (Fig. 1). It served as the aqueous phase in the SX experiments. Both leachate pH and speciation of the individual metals in the leachate play a significant role because this will determine which extractant(s) will be used: cationic exchanger (D2EHPA or DPPD under acidic conditions), solvating extractant (TBP and Cy923), or anionic exchanger (Ali336). Co, Fe, Ni, and Mn and to a minor extent also Cu and Al are the actual target metals of the extraction stage. Al and Fe are not valuable metals. But for being contained as minor components in the leachates (< 2 %), they must be removed efficiently as they interfere considerably with Co, Mn, and Ni separation. In order to design a process to be operated economically, the number of stages needs to be as few as possible. For the extraction efficiency of these six metals the initial pH of the leachate emerged crucial and was therefore considered in the course of process development. To avoid precipitation of Fe or Al hydroxides, the pH was kept between 0.3 and 3.0. As shown in Fig. 2, there is a complex interplay between sulfate salt formation, mixed salt formation, hydroxide formation, maturing to (mixed) oxides and precipitation, while all the various species of Al, Co, Cu, Fe, Mn, and Ni may be present as anionic, cationic, or neutral entities. Both parameters are directly related to the extraction mechanism and therefore, extraction reagent used in this stage. The concentrations of D2EHPA, TBP, Cy923, and Ali336 were fixed to 0.3 mol L−1, while DPPD was 0.1 mol L−1 with regard to its solubility in kerosene.
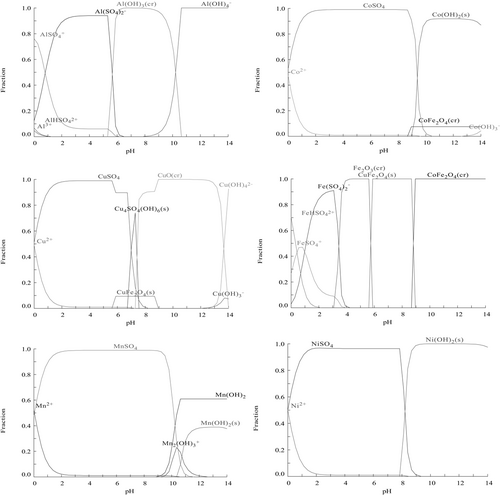
Fig. 3 shows the recovery results for the six metals with these five extractant reagents within a pH range between 0.3 and 3.0. The initial extraction experiment was done at pH = 0.3 which is the non-modified liquor obtained from sulfuric acid leaching of the Li-free black mass (0.5 mol L−1 H2SO4). It was carried out without any pH conditioning. In order to select the best conditions, initial pH and extractant used, a balance between the metal recovery and the separation factor has to be considered. This means that although with extractants such as TBP or Ali336, the recovery efficiencies are lower (< 70 %) compared to those obtained with cationic exchangers, the separation factor has also been considered since the main purpose is to reach a selective metal separation.
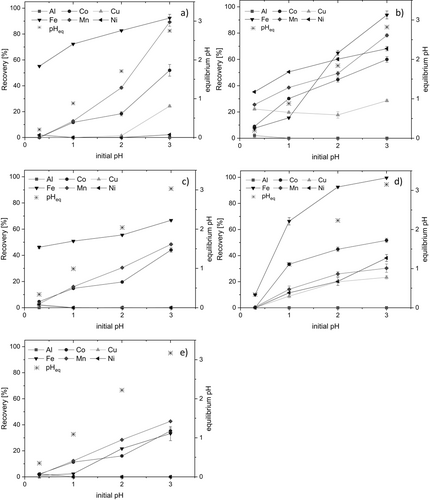
Ni, Co, and Cu have been reported to profit from higher pH values, though, when extracted with cationic exchangers 5, 6, 18, 21. Ni and Co can be recovered by solvating extractants, too, but the concentration of TBP or Cy923 should be increased as reported by Wang and Lee who used 1.5 mol L−1 of a mixture of Cyanex 301 and TBP 22.
At pH = 0.30 Fe recovery was 46.2 ± 1.01 % with TBP and 55.1 ± 0.21 % with D2EHPA, which is 19 % more. This is in line with similar results by Hu et al. for a chloride mixture of Fe/Ca/Mg/Ni/Co/Cu with D2EHPA 23. In fact, Fe separation is optimal with 0.3 mol L−1 of D2EHPA at pH = 0.3, where separation factors are highest (Tab. 2).
|
|
Initial pH |
βFe/Al |
βFe/Co |
βFe/Cu |
βFe/Mn |
βFe/Ni |
βMn/Al |
βMn/Co |
βMn/Cu |
βMn/Ni |
|---|---|---|---|---|---|---|---|---|---|---|
|
D2EHPA |
0.3 |
* |
* |
* |
* |
62.2 |
* |
* |
* |
0.01 |
|
1.0 |
* |
19.8 |
* |
18.0 |
* |
* |
1.1 |
* |
* |
|
|
2.0 |
* |
21.3 |
382 |
7.6 |
* |
* |
2.8 |
50.1 |
* |
|
|
3.0 |
0.3 |
12.7 |
40.5 |
1.5 |
594 |
0.2 |
8.4 |
27.2 |
* |
|
|
DPPD |
0.3 |
1.6 |
0.8 |
0.3 |
0.2 |
0.2 |
6.8 |
3.5 |
1.2 |
0.6 |
|
1.0 |
* |
0.4 |
0.8 |
0.3 |
0.2 |
* |
1.5 |
2.6 |
0.6 |
|
|
2.0 |
* |
2.3 |
8.6 |
1.9 |
1.2 |
* |
1.2 |
4.5 |
0.6 |
|
|
3.0 |
* |
11.9 |
44.2 |
4.9 |
8.4 |
* |
2.4 |
9.0 |
1.7 |
|
|
TBP |
0.3 |
* |
17.7 |
56.0 |
27.8 |
9.7 |
* |
0.6 |
2.0 |
0.4 |
|
1.0 |
* |
6.0 |
* |
5.4 |
* |
* |
1.1 |
* |
* |
|
|
2.0 |
* |
5.1 |
466 |
2.8 |
* |
* |
1.8 |
163 |
* |
|
|
3.0 |
* |
2.6 |
* |
2.1 |
* |
* |
1.2 |
* |
* |
|
|
Cy923 |
0.3 |
* |
* |
* |
11.3 |
* |
* |
* |
* |
* |
|
1.0 |
* |
4.0 |
20.9 |
12.1 |
15.3 |
* |
* |
1.8 |
1.3 |
|
|
2.0 |
* |
15.5 |
50.6 |
36.3 |
51.0 |
* |
0.4 |
1.4 |
1.4 |
|
|
3.0 |
* |
481 |
1700 |
1226 |
782 |
* |
0.4 |
1.4 |
0.7 |
|
|
Ali336 |
0.3 |
* |
0.6 |
1.1 |
0.7 |
0.5 |
* |
0.8 |
2.5 |
0.8 |
|
1.0 |
* |
0.2 |
* |
0.2 |
* |
* |
1.1 |
* |
* |
|
|
2.0 |
* |
1.5 |
* |
0.7 |
* |
* |
2.1 |
* |
* |
|
|
3.0 |
* |
0.9 |
* |
0.7 |
* |
* |
1.4 |
* |
* |
- * > 5000
The theoretically required stages to separate Fe from the mixture using a counter-current or cross-flow solvent extraction process are provided by a McCabe-Thiele diagram once the extraction equilibria are known and the mass balance equations at all stages are solved. Considering chemicals consumption as well as industrial feasibility, the counter-current process was more effective than cross-flow.
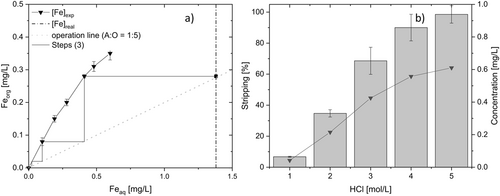
As shown in Fig. 4a, Fe can be eliminated from the aqueous phase by at least three stages of solvent extraction under an A/O ratio of 1:5. Iron separation is always in issue when separating polymetallic mixtures, and the same applies here. However, it has turned out that there is no general protocol available. Even worse, each individual separation problem requires an individual solution. At least, as a common feature, solvating extractants have emerged more powerful than cationic exchangers.
How complex this issue is, can nicely be illustrated by the following examples. For instance, the selective recovery of Fe was achieved by adding 2-octanol to an organic phase made from TBP and kerosene 24. However, Yi et al. suggest working in chloride media to remove Fe from Fe/Ni/Co mixture by two-step extraction process. Fe was individually separated from Fe/Co/Zn mixture in presence of chloride and sulphate ions by two counter-current stages using 1 mol L−1 TBP + 20 % methyl isobutyl ketone (MIBK) at an A/O ratio = 1:1 25. Cy923 was also used to selective recovery of Fe from Fe-Cr mixture from a tannery filtrate since FeCl3 was the predominant species, which gets extracted by this solvating extractant 26. The advantage of working with organic extractants such as TBP and Cy923 over D2EHPA is the lower concentration of stripping solutions (0.25 mol L−1 H2SO4) for re-extracting the metal from the loaded phase. In a nutshell, Fe separation did not succeed reasonably with TBP or Cy923 under the studied conditions in the present work. Not less than 5 mol L−1 HCl is required to strip Fe from the loaded phase (Fig. 4b). The concentration of the stripping reagent was reduced by at least 50 % compared to the one suggested by El-Nadi & El-Hefny 27. 1.2 mol L−1 H2SO4 were required to strip Mn and Cd with D2EHPA 30 % at pH = 2.5 28.
Considering the aim of the selective separation of the Al, Co, Cu, Mn, and Ni mixture, it can be seen in Fig. 3 that 0.1 mol L−1 DPPD is not suitable for establishing a cost-efficient separation process. The results are according to those reported by Pavón et al. who had demonstrated that a similar extractant (2,4-pentanedione) is selective, but for rare earth elements 29. It had turned out during these investigations that keto-enol tautomery exerts a decisive effect on extraction efficiency. DPPD is protonated at lower pH, and the proton can be replaced by the metal to be extracted (Fig. 5).
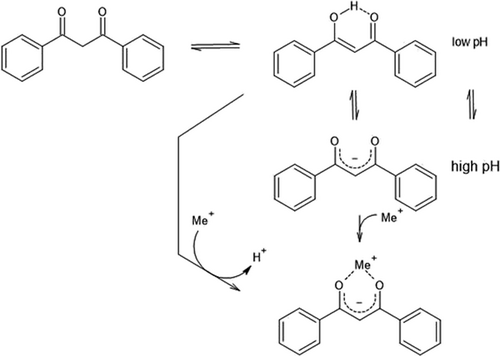
Under these conditions the extraction mechanism can be conceived of as comparable to D2EHPA. Both species formally behave like Brønstedt acids, where DPPD can be understood as a vinylogous carboxylic acid. Deprotonation yields the enolate anion, which through its higher electron density serves well as a complex forming agent. For the purposes of metal extraction however, DPPD is efficient for metal recovery, but it lacks the discriminatory power to selectively separate any of the metals studied here.
Considering that TBP and Cy923 are solvating extractants, the recovery percentage of any metal is expected to be maintained regardless of the initial pH of the aqueous phase 30. However, since these extractants are capable of complexing protons, too, the number of available binding sites is reduced 31. Thus, their extractory power may be less under these conditions. As a result, extraction efficiencies increase with increasing pH. Furthermore, at pH 2-3, the neutral species are favored (Fig. 2), what in turn increases extraction efficiency for all metals studied.
According to the results depicted in Fig. 3, Mn can be separated from the mixture by TBP since the separation factors are higher compared to Cy923 (Tab. 2). It has been reported that Mn is selectively separated from a mixture of Mn-Zn because of the synergistic effects obtained from a mixture of TBP and Cyanex 301 32. However, the concentration of the sulphate media is at least 10 times lower than the one used in the current work. They also demonstrated that this organic mixture extracts protons, too, due to the low initial pH value. D2EHPA as a cationic exchanger can be also used for this purpose 28, but the extractant consumption was 3 times higher (0.9 mol L−1) compared to the one used in the current work.
It is evident that under these conditions of pH and extractant, Co will be co-extracted, too, but afterwards the mixture of Mn-Co should undergo a further individual separation process 33. Investigation of selective stripping reagents is also a possibility to be evaluated in further research. Although Mn separation can be achieved at pH = 2 or 3, less stages are required at pH = 3, where Mn recovery reached 48.4 ± 0.65 % (Fig. 3). Considering the 10-times higher Mn concentration in the solid residue (Tab. 1), the extraction isotherm was investigated referring to Mn (instead of Co). The McCable-Thiele plot (Fig. 6a) shows that at least three stages are necessary to reach full separation of Mn from the aqueous mixture phase at A/O ratio of 1:4.
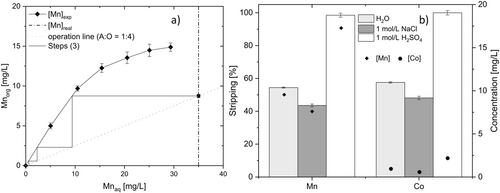
According to literature, water, NaCl and H2SO4 were chosen as stripping solution for re-extracting Mn from the organic phase into the aqueous phase, when TBP was used as an extractant 16, 34-36. The stripping experiments were carried out with an A:O ratio of 1, and the results are depicted in Fig. 6b. All three reagents recover manganese from the loaded phase. However, more than one stage is needed to achieve full metal recovery when water and 1 mol L−1 NaCl is used. Similar results were reported by Sarangi et. al. needing three counter-current stages with an A/O ratio of 1:2 to recover 11.8 kg m−3 iron 25. With 1 mol L−1 H2SO4, the Mn stripping reaches 98.5 ± 1.02 % in one stage.
Ali336 as anionic exchanger was also studied to investigate the selective separation of the Al, Co, Cu, Fe, Mn and Ni mixture. Nevertheless, the recovery efficiencies are similar for all these metals (Fig. 3). Chloride is replaced for other anions in the extraction mechanism for this kind of extractant. As depicted in the MEDUSA® diagrams, under these pH conditions, anionic species are not favored. Therefore, metal recovery is < 12 % between pH = 0 and 1. When anionic species such as HSO4− or SO42− are present in the system (pH > 2), the recovery of all metals is increased. However, it can be also assumed that in the case of SO42−, two molecules of Ali336 are required for 1 molecule of this anionic species. As a result, the concentration of Ali336 should be increased to reach higher efficiencies or work under chloride or nitrate media instead of sulphate one 37.
3.2 Separation Process Proposed
The flowsheet proposed for separating the metals contained in the solid residue subsequent to the COOL-process is depicted in Fig. 7. The digestion of this residue was carried out using 0.5 mol L−1 H2SO4, hence the leachate pH was 0.3. Without any pH conditioning, > 98.5 ± 0.65 % Fe was recovered by a three-stages counter-current process using 0.3 mol L−1 of D2EHPA diluted in kerosene with a A/O ratio = 1:5. This metal was fully stripped from the loaded phase using 5 mol L−1 of HCl (same A/O ratio) to reach a concentration of Fe of 1.35 mg L−1. D2EHPA can be used in further runs after the scrubbing step to clean properly the extractant avoiding loss of efficiency. To separate Mn/Co mixture from the aqueous solution (Fe-free), the pH value has to be increased to 3.0 by adding NaOH. TBP as solvating extractant was used with a concentration of 0.3 mol L−1 in a four-stages counter-current solvent extraction process with A/O = 0.25 reaching ≥ 99.9 ± 1.11 % of Mn with full co-extraction of Co (99.8 ± 0.78 %). Four stages instead of three were applied to avoid 0.74 mg L−1 Co as an impurity in the Ni/Cu mixture flow. The selective separation of this mixture requires further work. For instance, one option is finding selective conditions in the stripping process. This possibility will be addressed in follow-up research.
Mn, as well as Co, were stripped using a single stage from the loaded phase using 1 mol L−1 of H2SO4 in an A:O ratio of 1. The concentration of both metals in the stripping solution were 38.3 ± 0.91 mg L−1 of Mn and 3.7 ± 0.12 mg L−1 of Co. The scrubbing step was required to recirculate the extractant saving chemical consumption and contributing to a circular economy. Afterwards, 1.2 mg L−1 of Al (≥ 99.7 ± 1.07 %) was recovered as Al(OH)3 (Fig. 2) at pH > 5.0. For this reason, the aqueous solution was conditioned increasing the pH value until 6.0. After filtration, a Ni/Cu mixture is obtained with 14.4 ± 2.1 and 3.1 ± 0.48 mg L−1 of Ni and Cu (98.6 ± 1.32 % Ni; 97.8 ± 1.46 % Cu), respectively, the separation of which can be done selectively by different techniques such as electrodeposition 38, aqueous two-phase system (ATPS) formed by copolymers 39, solvent extraction using Chemorex CP-150 40, or else. However, it is evident that the initial conditions should be modified depending on which is the selected technique to reach this separation purpose.
The complete separation process needs to be evaluated from a technical, environmental, and economic point of view to contribute to a cost-efficiency process development. In this sense, adding supported liquid membrane technique, not only the chemicals consumption will be reduced but also the option to concentrate the aqueous flows in accordance with hollow fiber module configuration. Furthermore, the membrane techniques are more efficient compared to the solvent extraction process when the concentration of the metals is low 41, 42. Therefore, optimization of this process is matter of ongoing research.
4 Conclusions
The current work shows how the remaining metals in the solid residue obtained from the COOL-Process can be separated individually. After digestion of this solid residue with 0.5 mol/L of H2SO4, full recovery of Al (1.2 ± 0.02 mg L−1; 99.7 ± 1.07 %), Co (3.7 ± 0.12 mg L−1; 99.8 ± 0.78 %), Cu (3.1 ± 0.48 mg L−1; 97.8 ± 1.46 %), Fe (0.8 ± 0.21 mg L−1; 98.5 ± 0.65 %), Mn (38.3 ± 0.91 mg L−1; 99.9 ± 1.11 %), and Ni (14.4 ± 2.1 mg L−1; 98.6 ± 1.32 %) was achieved. Fe was selectively recovered from the mixture by a counter-current process with three-stages using 0.3 mol L−1 of D2EHPA with a A/O ratio = 1:5. Fe was stripped from the loaded phase with 5 mol L−1 of HCl. A mixture of Mn/Co was recovered from the free-Fe solution after a four-stages counter current process, previous basification to fix the initial pH to 3.0, using 0.3 mol L−1 TBP as an extractant (A:O = 0.25). Both metals were re-extracted from the loaded phase into the stripping solution (1 mol/L H2SO4). Increasing the pH of the aqueous phase to 6.0, the Al is separated by precipitation of Al(OH)3, obtaining after filtration a mixture of Ni/Cu. Further research will be carried out to achieve the separation of Mn/Co and Ni/Cu mixtures. Furthermore, the possibility to apply membrane techniques instead of solvent extraction process will be investigated as well to reduce the chemicals consumption. Although the complete recycling process of LIBs has to be further evaluated from a technical, environmental, and economic point of view, it has been demonstrated with the present work that with a holistic recycling process not only Li as Li2CO3, but also the other contained metals such as Al, Co, Cu, Fe, Mn, and Ni can be recovered from LIB black mass with high discriminatory power, thus considerably contributing to the aims of circular economy.
Acknowledgements
Financial support by the German Federal Ministry of Education and Research (Grant nr. 033RC020A) is gratefully acknowledged. Further thanks are owed to Andrea Schneider, Ulrike Börner and Kim Meerbach for conducting ICP-OES and solvent extraction experiments. The authors would like to thank to RMF GmbH, Freiberg, Germany for providing LIB black mass sample. Open access funding enabled and organized by Projekt DEAL.
Symbols used
-
- %E %
-
extraction efficiency
-
- [Fe]exp [mg L−1]
-
experimental iron concentration
-
- [Fe]real [mg L−1]
-
real iron concentration
-
- [Me]0 [mg L−1]
-
initial metal concentration
-
- [Me]aq [mg L−1]
-
equilibrium metal concentration
-
- [Mn]exp [mg L−1]
-
experimental manganese concentration
-
- [Me]org [mg L−1]
-
concentration of the metal in the loaded organic phase
-
- [Mn]real [mg L−1]
-
real manganese concentration
-
- [Me]str [mg L−1]
-
metal concentration in the stripping phase
-
- %S %
-
stripping efficiency
-
- Vaq [L]
-
volume of the aqueous phase under initial, equilibrium and stripping conditions
-
 [L]
[L] -
volume of the organic phase
Greek letters
-
- β
-
separation factor
Abbreviations
-
- A/O
-
aqueous/organic
-
- Cy572
-
Cyanex 572
-
- Cy923
-
Cyanex 923
-
- D
-
distribution ratio
-
- D2EHPA
-
bis(2-ethylhexyl)phosphoric acid
-
- DPPD
-
1,3-diphenyl-1,3-propandione
-
- LIB
-
lithium-ion battery
-
- SX
-
solvent extraction
-
- TBP
-
tributyl phosphate




