Recovery of Al, Co, Cu, Fe, Mn, and Ni from Spent LIBs after Li Selective Separation by the COOL-Process. Part 1: Leaching of Solid Residue from COOL-Process
Abstract
Lithium recycling from spent LIBs along the COOL-process produces a Li-free metal rich black mass, which still contains the entire fraction of valuable metal such as Co, Ni, and Mn. A recycling process was developed, which allows for mobilizing these metals. The first process stage is the selective leaching of Li via COOL-process. Subsequently, two inorganic (H2SO4 and HCl) and two organics acids (citric and gluconic acid) were tested for mobilizing the metals from the solid residue. To optimize this process stage, acid concentration as well as addition of H2O2 as a reduction reagent were investigated. The optimal conditions to dissolve valuable metals such as Co, Cu, Fe, Mn, and Ni was identified as 2 N H2SO4 and 2 vol % H2O2.
1 Introduction
The annual demand for lithium-ion batteries (LIBs) is steadily increasing, which is mainly due to their wide range of applications. Besides portable electronic devices (e.g., smartphones, tablets, video cameras), E-vehicles and grid storage are the main application fields.
Compared to other battery systems (e.g., nickel cadmium batteries, nickel metal hydride batteries, and lead-acid batteries), LIBs have several advantages such as high working voltage, large energy density, no memory effect, low self-discharge rate, and long cycle life 1. The rapid growth in the use of LIBs requires a significant amount of valuable metals such as cobalt, copper, lithium, manganese, and nickel. Their reserves are limited and unevenly distributed geographically. The resulting dependence on imports is spurring efforts to find innovative and effective methods to make them available. Furthermore, the constant introduction of new technologies and shorter lifetime of electronic devises, increase the disposal number of spent LIBs. Moreover, it is expected, that the mandatory recycling rates of LIBs will be further increased (currently in the EU: 50 wt % of the hole LIB) 2. Thus, innovative recycling approaches, which allow for recovering valuable metals are required to contribute to a circular economy and to develop an economically viable zero-waste solution.
The most established recycling processes for spent LIBs focus on recovering cobalt. The other valuable metals such as lithium, nickel, and manganese play a minor role what is owed to cost-efficient approaches lacking. And they are far cheaper available from primary sources than cobalt.
For metal recovery from LIB both hydrometallurgical and pyrometallurgical approaches are in use, such as Accurec and Umicore (pyrometallurgical) or Recupyl and GEM (hydrometallurgical).3 Hydrometallurgical processes commence with digesting spent LIBs by inorganic or organic acids. Subsequently, a complex route of process steps provides the individual metals. Typical operations are precipitation or extraction. Hydrometallurgical routes are characterized by large amounts of fresh water needed and wastewater produced, the treatment of which is cost-intensive. In addition, high purity chemicals are required to ensure high product quality. Pyrometallurgical processes, on the other hand, are characterized by high energy consumption and investment costs are high. In addition, hazardous gases are produced, in particular from fluorine containing battery components. In the case of LIB isolating the individual metals is rather complex and lithium remains in the slag, from where its recovery is difficult or even impossible 4-7. This does not mean that hydro- or pyrometallurgical processes are inapt, yet just now, neither route is sufficiently well developed to be operated satisfactorily from the viewpoint of process economy and sustainability. Present initiatives, such as the EarLiMet-approach aim at a cost-efficient adaptation of pyrometallurgical pre-processing combined with wet-chemical metal recovery 8.
It is characteristic for contemporary process development, that recycling from secondary sources typically implies routes highly distinct from primary raw material processing. As a consequence, parallel industries are established aiming at parallel or incompatible markets. It has to be noted, though, that recycling means nothing else but re-establishing primary product quality. In other words, a technology is required that does not differentiate between primary and secondary lithium feedstock, one that is origin independent. This by the way is the concept of circular resources chemistry (in German: Wertstoffchemie) 9. In fact, a solution to solving future challenges in raw material processing lies in designing such hybrid processes, which are capable of digesting both primary and secondary sources along one single process chain. Only the entry points are different.
A textbook example for such approaches is lithium recovery from black mass by the COOL-process, which had been proven unparalleled effective in processing zinnwaldite, a lithium ore 10. Here, after separating and recovering housing materials, copper and polymer foils, the black mass is treated with supercritical (sc)-CO2, which allows for leaching Li selectively, providing an aqueous solution of LiHCO3. Under optimal conditions (T = 230 °C, t = 4 h, water/black mass ratio = 90 mL g−1, P = 10 MPa), 98.6 wt % lithium was recovered as lithium carbonate (Li2CO3). Already the crude product has battery grade-quality (> 99.8 wt %), i.e., there is no further refining needed. The other valuable metals Co, Cu, Mn, and Ni remain in the solid residue 2.
Sustainable use of LIBs means closing the loop, thus full recovery of all valuable metals. The aim of the present work is therefore to digest the solid residue with acids and then isolate the dissolved metals by solvent extraction (Fig. 1). The overall study on this topic is divided into two parts. The aim of this work (Part 1) is the investigation of the solid residue digestion stage. Subsequent work (Part 2 23) comprises the selective separation of the valuable metals, which will be comprehensively investigated by using solvent extraction processes.
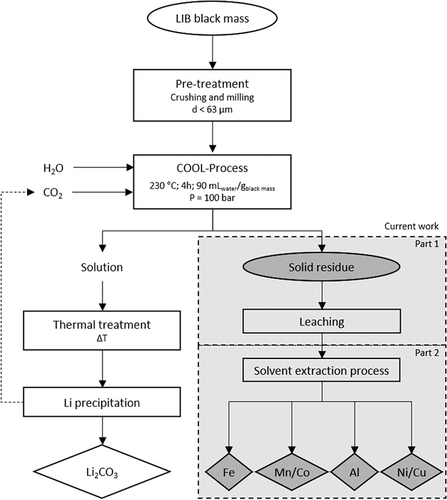
2 Material and Methods
2.1 Materials
LIB black mass samples were provided by RMF GmbH (Freiberg, Germany). The recycling process depicted in Fig. 1 had previously been developed and optimized for obtaining Li2CO3 by the COOL-process 2. In the present work, the starting material is the solid residue obtained after the COOL process, which is after leaching black mass with sc-CO2. The black mass samples were of the same origin. The composition of the combined solid residue is depicted in Tab. 1.
|
|
Al |
Co |
Cu |
Fe |
Mn |
Ni |
|---|---|---|---|---|---|---|
|
Composition [wt.%] |
2.01 ± 0.11 |
2.81 ± 0.01 |
2.69 ± 0.08 |
0.38 ± 0.004 |
30.18 ± 0.74 |
9.81 ± 0.10 |
The solid residue's elemental composition was determined by atomic absorption spectroscopy (AAS, ContrAA 700, Analytik Jena, Jena, Germany) and atomic emission spectrometry with inductively coupled plasma (ICP-OES, Optima 4300 DV, Perkin Elmer, MA, USA). The solid sample was leached with aqua regia in a liquid/solid ratio (L/S) of 100 at 180 ± 2 °C for 30 min using a Microwave MARS 6 device (CEM Corporation, London, Great Britain). The procedure was carried out in duplicate.
2.2 Leaching
The solid residue obtained from the COOL-process, which was carried out according to the optimized parameters described by Pavón et al. (230 °C for 4 h with a water/black mass ratio of 90 mL g−1) 2, was treated in a first digestion stage with different leaching reagents with a concentration of 2 N Sulfuric acid (95 %, Thermo Fischer Scientific, MA, USA), hydrochloric acid (37 %, VWR International, PA, USA), citric acid (99+ %, Alfa Aesar, MA, USA), and gluconic acid (50 %, Sigma-Aldrich, MO, USA) as well as different concentrations of hydrogen peroxide (0–6 vol %; 30 %, Th. Geyer, Renningen, Germany) were tested. After the first screening, the concentration of sulfuric and hydrochloric acid was optimized. For these purposes, acid concentration was varied in the range between 0.2 and 4 N at a constant hydrogen peroxide concentration of 2 vol %. For all leaching experiments the solid residue (10 wt %) was mixed with the leaching reagent at 50 °C for 2 h and 100 rpm in an incubation shaker (INFROS HT, Bottmingen, Switzerland). After leaching treatment, the suspensions were filtered, and the solid residues were washed with deionized water and dried. All leaching tests were done in duplicate. The filtrate was diluted by factor 10 and analyzed by ICP-OES.
3 Results and Discussion
A traditional treatment for recovering valuable metals from ashes, ores, biomass or other solid materials is digesting stage with inorganic or nowadays increasingly organic acids. Mineral acids such as sulfuric acid, hydrochloric acid, and nitric acid usually show higher leaching efficiencies. However, these acids also have disadvantages, such as the release of toxic gases or strong corrosiveness 11. For this reason, organic acids such as citric acid, oxalic acid or tartaric acid are progressively used for leaching of spent LIBs. They profit from being perceived of as environmentally more benign for their biodegradability combined with lower corrosiveness. In addition, organic acids may act as reducing agents, which can further increase leaching performance 5. On the other hand, they are far more costly and their use can only be justified in terms of process economy by the intrinsic value of the metal content exceeding the value of the reactants used 11.
3.1 Screening of Different Acids
Based on an extensive literature search, hydrochloric acid, sulfuric acid as mineral acids, and citric acid as an organic acid emerged as promising leaching agents. Gluconic acid was chosen as an additional digestion agent for being slightly corrosive and environmentally highly compatible.12 From the use of gluconic acid, which has been little investigated in LIB recycling before, we expected insight in potential bioleaching approaches in this field.
Screening these four selected acids (Fig. 2) showed that the highest leaching efficiency (39.7–87.7 %) is obtained for hydrochloric acid. It was the best leaching reagent for all studied metals. This observation is known from literature, too, where it is explained by the high acidity of HCl. Of course, this explanation alone is not sufficient, since the Hammett acidity of H2SO4 is far higher 13. It is also the corrosive Cl− anion which acts as a reductant for M3+ and M4+ 14. For instance, at low temperatures, Co2+ is much more soluble than Co3+ 4, 15. Since cobalt is usually firmly bound as Co3+ in black mass, it must be reduced prior to getting dissolved. This explains why with sulfuric acid only 43.5 % cobalt is mobilized, whereas with hydrochloric acid 73.9 % is leached. Sulfuric acid gave leaching efficiencies that did not exceed 46.6 %. Urbańska made similar observations under comparable conditions, with leaching efficiencies even lower (≤ 35 %) 11. Whether or not organic acids perform better, is matter of controversial discussion. Aaltonen et al. for instance report higher leaching efficiencies with the mineral acids HCl and H2SO4 instead of organic acids 16. Citric acid only mobilized copper and manganese at acceptable concentrations (Cu 59.6 %, Mn 71.4 %).
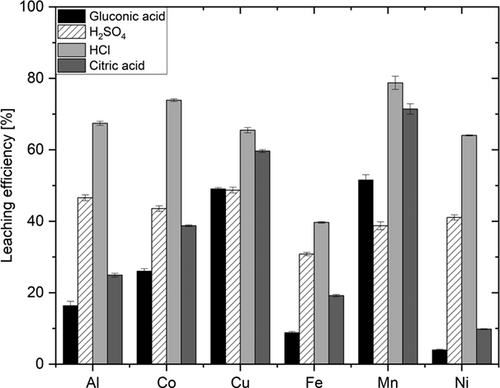
The leaching efficiency for Cu and Mn is higher than that of sulfuric acid and only 5.9 and 7.3 percentage points lower than that of hydrochloric acid. This can be explained by the fact that citric acid, like hydrochloric acid, displays reducing properties. It is not clear, though, whether it is these reductive properties or the complex forming abilities or a combination of both is responsible for the observed effects. Gluconic acid gave only insufficient mobilization rates except for copper and manganese. Its leaching efficiency for copper is almost identical to that obtained with sulfuric acid (49.1 vs. 48.7 %). In the case of manganese, gluconic acid even gave an efficiency 12.9 percentage points higher than sulfuric acid. The leaching efficiency of iron is < 39.7 % and thus, insufficient. It can be assumed that the iron content was introduced during the mechanical processing of the spent LIBs. Thus, it is elemental iron which is difficult to dissolve under the mild reaction conditions used.
The use of hydrochloric acid has some disadvantages despite its good leaching efficiency. Above all stands the fact that toxic Cl2 gas is formed and released by the oxidation of Cl−. Furthermore, hydrochloric acid is very corrosive, which entails high material requirements and thus high investment costs 6, 14. For these reasons, it was investigated to what extent the addition of reducing agents would increase the leaching efficiency.
3.2 Effect of Hydrogen Peroxide
Many studies have shown that adding reducing agents is necessary to render leaching efficient. This is especially owed to the fact that the contained cobalt is present as Co3+, which is difficult to dissolve at low temperatures. The reduction of Co3+ to Co2+ significantly improves the leaching efficiency as well as the reaction kinetics. The same was observed for manganese. A frequently used reducing agent is hydrogen peroxide, but other reducing agents such as ascorbic acid and glucose have also been tested 4, 6, 17. Hydrogen peroxide is the most commonly used reductant due its low cost, low oxidation potential and the production of the harmless by-product water 6, 18, 19. The reduction potential of hydrogen peroxide allows for reducing Co3+ to Co2+ as well as Mn4+ to Mn2+, which improves their solubilities compared to the initial state 17. Many studies have shown the necessity for adding H2O2 in order to achieve efficient recovery of Li and Co from spent LiCoO2 batteries. Since the chemical bonds between cobalt and oxygen are extremely strong, leaching of LiCoO2 is difficult. The addition of hydrogen peroxide generates oxygen, which promotes cobalt oxide dissolution by reducing Co3+ to Co2+ 5. For other types of spent LIBs, containing Ni, Mn, Cu, and Fe in addition to Co and Li, there exist only few studies on the effect of H2O2. Since hydrogen peroxide can act both as a reducing and as an oxidizing agent, cross effects can take place in the presence of different metals, therefore the influence of H2O2 was investigated.
Fig. 3 shows the effect of the hydrogen peroxide concentration on leaching efficiency. In the case of sulfuric acid, it assumes the highest value for all metals when using 2 vol % hydrogen peroxide. In comparison to leaching without hydrogen peroxide addition, the leaching efficiency increase in a range of 20.1 to 43.8 %. Manganese mobilization was even more than doubled. This shows the importance of reducing the bound manganese to Mn2+ to improve its solubility. Further increasing hydrogen peroxide concentration however takes a slightly negative effect on efficiency. This finding is in line with Aaltonen et al. who determined 2 vol %. hydrogen peroxide as the optimum concentration for leaching spent LIBs with sulfuric acid too, but at 70 °C and 5 h leaching.
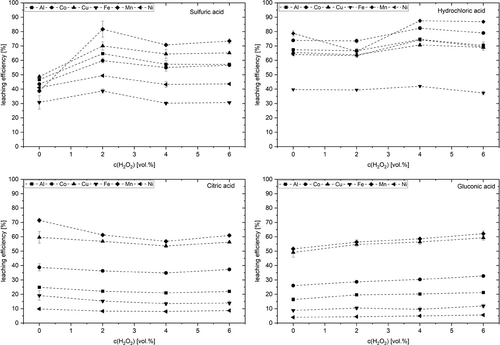
However, their experiments were carried out at 70 °C 16. Nayl et al. observed the same progression for Co, Mn, and Ni, with the maximum reached at 4 vol % 19. Wang et al. also observed a maximum cobalt mobilization at the addition of 6 vol % hydrogen peroxide and a subsequent decline of the mobilization at further increase of reducer concentrations 1. The decrease in mobilization after reaching a maximum is explained by the decomposition of the hydrogen peroxide at increasing concentrations, as well as a decreasing reduction potential 1, 16.
The addition of hydrogen peroxide to hydrochloric acid achieved only a small increase in leaching efficiency. One can summarize the state of the art as somewhat diverging and non-uniform. Only for nickel a clear effect was observed. The addition of 4 vol % hydrogen peroxide increased the leaching efficiency by ten percentage points. As mentioned above, HCl already has a reducing effect on multivalent metals, therefore addition of a reducing agent is not necessary. The same applies to citric acid, where however it is not clear to which extent complex formation contributes to shifting equilibria to the right. In the case of manganese, the situation is as adverse as in the case of Co. Here, a mobilization was observed to be even 14.7 % lower. In contrast, Chen at al. reports a significantly improved leaching efficiency for Co from 48 to 85 %, for Mn from 43 to 80 %, and for Ni from 51 to 85 % with the addition of 2 vol % hydrogen peroxide. But these leaching tests were conducted at 80 °C 20. In the case of gluconic acid, the leaching efficiency increases with increasing concentration of hydrogen peroxide for all the metals investigated. Nevertheless, the determined improvements for Al, Co, Cu, and Mn are below 29.3 %. Only the leaching efficiency of Fe and Ni is increased by 34.6 and 38.8 % by adding hydrogen peroxide.
For leaching of black mass from LiFePO4 batteries with acetic acid and H2O2, Yang et al. observed the expected oxidation of Fe2+ to Fe3+, which in turn however decreased leaching efficiency 21. Similar findings were made by Li et al. during leaching of LiFePO4 with sulfuric acid 18. Since the Fe content in the current work was very low (0.38 wt %, Tab. 1), the oxidation of Fe2+ to Fe3+ plays a minor role, only. As such, no significant effect on iron leaching behavior was observed. Furthermore, the iron most likely originated from mechanical pre-processing of the batteries, so that the contained iron is either elemental or as Fe3+.
The mobilization rates of gluconic and citric acid were significantly lower than those obtained with mineral acids, for which reason they were not further investigated. Hydrochloric acid and sulfuric acid gave comparable results, while nitric acid released nitric oxides, our work focused on these two acids. With respect to a large-scale application, acid consumption is crucial for process economy. Further work therefore aimed at reducing acid use.
3.3 Effect of Acid Concentration
Fig. 4 illustrates how hydrochloric acid concentration affects leaching efficiency. For Al, Co, Fe, and Ni, leaching performance profits from increasing acid concentration. In the case of Cu, increasing acid concentration from 0.2 to 1 N results in elevating copper mobilization from 11.1 to 79.8. However, a further increase up to 4 N reduces the value by 20 % down to 63.8 %. A similar behavior is observed for Mn, where the highest leaching efficiency (82.9 %) was obtained with 2 N HCl, too.
On first sight the difference between 1 and 2 N HCl is meagre (1.1 percentage points), and an acid concentration of 1 N appears preferable for economic reasons, in terms of process effort however, it would make no sense mobilizing at different pH. The compromise to best possibly recover all valuable metals is 4 N. The same results were also obtained by Wang et al. They also determined 4 N HCl as the optimum concentration for leaching Co, Mn and Ni from spent LIBs 22.
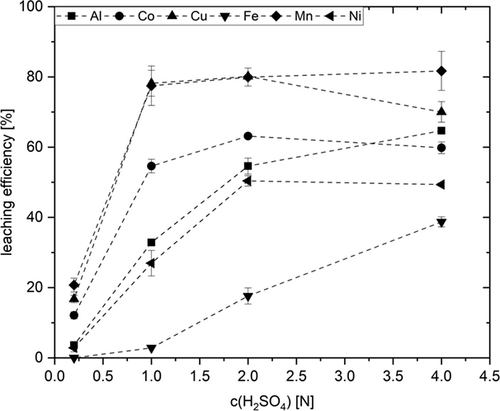
For sulfuric acid the situation is somewhat different. Being even more acidic, an increased H2SO4 concentration results in a better leaching efficiency for all investigated elements (Fig. 5). Leaching efficiency is already maximal at 2 N H2SO4 for Cu and Mn. However, Ni and Al would profit from the same compromise value 4 N. This however needs to be discussed in the light of the absolute amounts of Al and Fe present. In fact, only Ni would profit from higher acid strength. These results are in line with the findings by Nayl et al., who determined 2 N H2SO4 as optimal acid concentration for leaching Co, Mn, and Ni from spent LIBs. In contrast to the present work, their maximum Ni leaching was reached at 1.5 N 19. This observation is likely to be attributable to the higher reaction temperature (70 °C), as well as the higher H2O2 concentration (4 vol %) in that report.
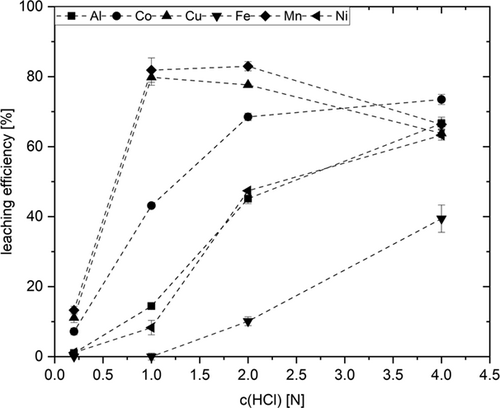
An issue which remains to be solved is the adverse impact of high acid strength on Cu and Mn yield for both acids. Since both metals are redox active, it may be hypothesized that valence changes affect their solubility and thus their concentration. Elucidating the exact reasons, the interaction with other redox active species such as Co(II/III), Fe(II/III), or H2O2 is matter of ongoing studies. The copper contained in the black mass is present in elemental form. When adding H2O2, oxidation to Cu2+ occurs on first place, what renders copper in water-soluble form. With increasing acid concentration however, Cu2+ reacts with elemental copper to give poorly soluble monovalent Co+ 22. In the case of manganese, the formation of sparingly soluble oxides cannot be ruled out. Furthermore, there may result Cu and Mn losses through adsorbing on the carbon contained.
The leaching results for Co, Cu, Mn, and Ni at optimized acid concentrations are summarized in Tab. 2. As can be seen there, the effect of the acid chosen on cobalt mobilization is poor. Roughly the same applies to copper and manganese. Only in the case of nickel, leaching with HCl was somewhat more distinct. Compared to many studies, the leaching efficiencies achieved are lower. This is in particular due to the fact that a comparatively low temperature of 50 °C was selected. Many leaching tests were carried out at 70–90 °C. Since hydrogen peroxide is not stable at this temperature, a lower temperature was chosen. Adsorption of the dissolved metals on graphite is conceivable, too.
|
|
|
|
|
Concentration in leaching solution [mg g−1solid residue] |
|
|||
|---|---|---|---|---|---|---|---|---|
|
Acid |
Co |
Cu |
Mn |
Ni |
||||
|
c [mg g−1] |
η [%] |
c [mg g−1] |
η [%] |
c [mg g−1] |
η [%] |
c [mg g−1] |
η [%] |
|
|
2 N H2SO4 |
17.8 |
63.2 |
21.6 |
80.1 |
241.3 |
79.9 |
49.4 |
50.4 |
|
4 N HCl |
20.7 |
63.8 |
17.2 |
63.8 |
200.3 |
66.4 |
62.1 |
63.3 |
To cut a long story short, there is no optimal leaching agent and no optimal leaching condition that provides maximum yields. Slightly better or comparable leaching results have been obtained with sulfuric acid, for which in addition a lower concentration can be used compared to hydrochloric acid. With H2SO4 being available on the market at approx. half the price of HCl, there is another pro in favor of this acid. The most decisive point however is not metal mobilization, but metal winning. If this is done by electrodeposition, chloride anions clearly impose an issue, while sulfate does not. The same applies for solvent extraction for metal recovery, where the use of sulfuric acid as a leaching reagent is an excellent starting point for workup, too.
4 Conclusion
A process has been developed which allows for mobilizing the valuable metals contained in black mass subsequent to Li recovery with the COOL-Process. In this direct carbonization, the black mass is first leached with supercritical CO2 to selectively mobilize lithium. The remaining solid residue is then subjected to an acid digestion stage, in which the contained metals are dissolved. A screening of two inorganic and two organic acids showed that the organic acids were not suitable to allow for efficiently recovering the metals contained in the black mass (< 62.2 %). The only exception was Mn for which 71.4 % mobilization were obtained with citric acid, possibly owed to supporting complexation. The addition of hydrogen peroxide as a reducing agent did not significantly alters leaching efficiency. Therefore, further optimizing acid concentration was carried out with hydrochloric acid and sulfuric acid. It was found that 2 N sulfuric acid or 4 N hydrochloric acid gave best leaching performances. In both cases, 2 vol % hydrogen peroxide were added as a reducing agent. Since both acids are almost comparable, in view of downstream processing as well as in terms of acid costs the use of sulfuric acid is recommended. Another benefit of H2SO4 becomes effective in the case of recovering the metals by electrodeposition, where the formation of toxic chlorine gas is avoided. Also, material and safety requirements are lower for sulfuric acid compared to hydrochloric acid, which in turn significantly reduces investment and operating costs. The sulphate metal solution generated is an ideal starting point for solvent extraction processes to recover the contained valuable metals. An increase in leaching efficiency can likely be achieved by elevating reaction temperature as well as extending reaction time. This and testing alternative reducing agents are matter of future investigations.
Acknowledgements
Financial support by the German Federal Ministry of Education and Research (Grant nr. 033RC020A) is gratefully acknowledged. Further thanks are owed to Andrea Schneider for conducting ICP-OES and Daniel Schmidt for carrying out the COOL-Process of the black mass. The authors would like to thank RMF GmbH for providing LIB black mass samples. Open access funding enabled and organized by Projekt DEAL.
Abbreviations
-
- LIB
-
lithium-ion batteries




